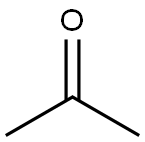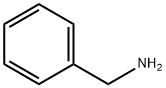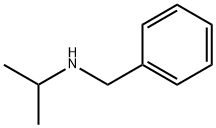
N-Isopropylbenzylamine synthesis
- Product Name:N-Isopropylbenzylamine
- CAS Number:102-97-6
- Molecular formula:C10H15N
- Molecular Weight:149.23
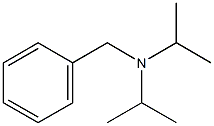
34636-09-4
48 suppliers
$18.00/1g

102-97-6
357 suppliers
$14.00/25g
Yield:102-97-6 86%
Reaction Conditions:
with iodine;sodium carbonate in chloroform at 60; for 24 h;
Steps:
Typical experimental procedure for removal of N-isopropyl group from N,N-diisopropylarylmethylamine:
General procedure: To a solution of N,N-diisopropyl-p-bromobenzylamine (1.0 mmol, 270.2 mg) in CHCl3 (2.0 mL) was added I2 (1.5 mmol,380.7 mg) and Na2CO3 (2.0 mmol, 212.0 mg) at room temperature, and the mixture was stirred for 24 h at 60 C. The reaction mixture was cooled to room temperature and quenched by satd aq Na2SO3 (10 mL), and extracted with CHCl3 (20 mL 3). Then, the organic layer was dried over Na2SO4. After removal of the solvent under reduced pressure, the yield was determined by 1H NMR analysis (89%). The residue was purified by short column chromatography on neutral silica gel (AcOEt/EtOH = 7:3) to afford N-isopropyl-p-bromobenzylamine.
References:
Ezawa, Masatoshi;Moriyama, Katsuhiko;Togo, Hideo [Tetrahedron Letters,2015,vol. 56,# 48,p. 6689 - 6692]
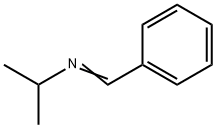
6852-56-8
23 suppliers
$106.00/250MG

102-97-6
357 suppliers
$14.00/25g
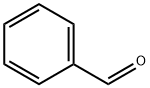
100-52-7
971 suppliers
$20.37/250g
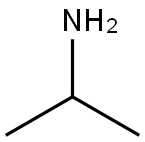
75-31-0
439 suppliers
$14.00/25mL

102-97-6
357 suppliers
$14.00/25g
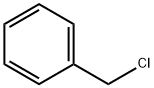
100-44-7
659 suppliers
$13.50/250G

75-31-0
439 suppliers
$14.00/25mL

102-97-6
357 suppliers
$14.00/25g