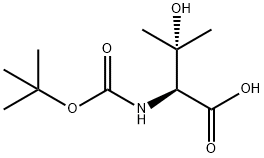
N-BOC-(S)-2-AMINO-3-HYDROXY-3-METHYLBUTANOIC ACID synthesis
- Product Name:N-BOC-(S)-2-AMINO-3-HYDROXY-3-METHYLBUTANOIC ACID
- CAS Number:102507-13-1
- Molecular formula:C10H19NO5
- Molecular Weight:233.26
![Carbamic acid, [2-hydroxy-1-(hydroxymethyl)-2-methylpropyl]-, 1,1-](/CAS/20180808/GIF/182958-73-2.gif)
182958-73-2
4 suppliers
inquiry

102507-13-1
134 suppliers
$43.00/100mg
Yield:102507-13-1 76%
Reaction Conditions:
with sodium dihydrogenphosphate in tetrahydrofuran at 0; for 4 h;
Steps:
1.2 Step 2: Preparation of (s)-2-tert-butoxyamido-3-methyl-3-hydroxybutyric acid
(s)-2-tert-butoxyamido-3-methyl-3-hydroxybutanol (20 g) was added to THF (50 mL). After adding saturated sodium dihydrogen phosphate solution (50 mL), the reaction was cooled to 0°C. Add bleach (6%, 350 mL) slowly. After 4 hours of reaction, slowly rise to room temperature. After TLC detects that the reaction is complete, it is quenched by adding sodium thiosulfate. Rotary evaporation to remove THF, then add dichloromethane (100mLX4) for extraction, dry and filter, concentrate to the crude reaction product, add n-hexane (100mL), beating and filtering to obtain pure product (s)-2-tert-butoxyamide -3-Methyl-3-hydroxybutyric acid (16 g, 76%).
References:
Zhejiang Kaipu Chemical Co., Ltd.;Luo Linfeng;Tan Xianghui;Yan Xiaodong;Shen Yipeng;Zhou Chen CN111909067, 2020, A Location in patent:Paragraph 0023-0025; 0028-0029
![Carbamic acid, [(1R)-2-hydroxy-1-(hydroxymethyl)-2-methylpropyl]-, 1,1-](/CAS/20180713/GIF/473545-40-3.gif)
473545-40-3
27 suppliers
$45.00/10mg

102507-13-1
134 suppliers
$43.00/100mg
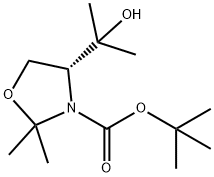
288159-36-4
1 suppliers
inquiry

102507-13-1
134 suppliers
$43.00/100mg
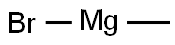
75-16-1
272 suppliers
$12.00/10ml
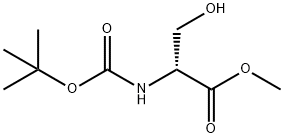
95715-85-8
241 suppliers
$5.00/1g

102507-13-1
134 suppliers
$43.00/100mg
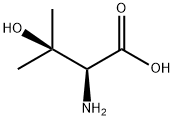
2280-27-5
101 suppliers
$77.00/100mg
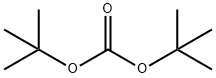
34619-03-9
28 suppliers
inquiry

102507-13-1
134 suppliers
$43.00/100mg