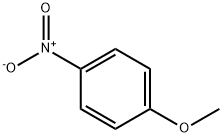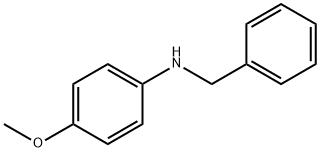
N-BENZYL-4-METHOXYANILINE synthesis
- Product Name:N-BENZYL-4-METHOXYANILINE
- CAS Number:17377-95-6
- Molecular formula:C14H15NO
- Molecular Weight:213.27
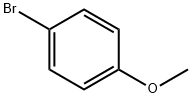
104-92-7
428 suppliers
$10.00/10g
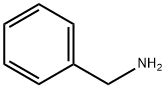
100-46-9
483 suppliers
$5.00/5 g

17377-95-6
81 suppliers
$58.00/5g
Yield: 98%
Reaction Conditions:
with copper(I) oxide;C18H18Br2N2O2;potassium hydroxide in dimethyl sulfoxide at 80; for 12 h;Inert atmosphere;Schlenk technique;Reagent/catalyst;Solvent;Temperature;
Steps:
29 Example 29
Example 29 Coupling Reaction of 4-bromoanisole with Benzylamine. Copper catalyst (0.01 mmol), ligand (0.01 mmol) and base (1.5 mmol) were added into a 10 mL of Schlenk tube. The tube was evacuated and backfilled with argon (three times), and then 4-bromoanisole (1.0 mmol), benzylamine (1.5 mmol) and 1 mL of solvent were added. The reaction mixture was stirred well at 80°C. for 12 hours. After cooling, water and ethyl acetate were added and mixture was separated. The aqueous phase was extracted twice with ethyl acetate. The combined organic phase was dried over anhydrous sodium sulfate. After concentration, the residue was purified by column chromatography to give the product N-(4-methoxy)phenylbenzylamine .
References:
CE Pharm CO., LTD;Ma, Dawei;Zhou, Wei;Fan, Mengyang;Wu, Haibo;Yin, Junli;Xia, Shanghua US10500577, 2019, B2 Location in patent:Page/Page column 123
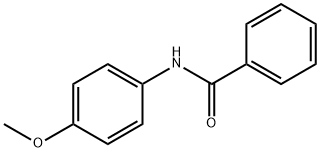
7472-54-0
62 suppliers
$12.00/1mg

17377-95-6
81 suppliers
$58.00/5g
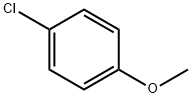
623-12-1
282 suppliers
$10.00/25g

100-46-9
483 suppliers
$5.00/5 g

17377-95-6
81 suppliers
$58.00/5g
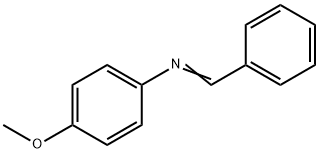
783-08-4
30 suppliers
$61.00/250mg

17377-95-6
81 suppliers
$58.00/5g