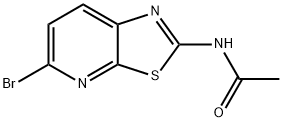
N-(5-BroMothiazolo[5,4-b]pyridin-2-yl)acetaMide synthesis
- Product Name:N-(5-BroMothiazolo[5,4-b]pyridin-2-yl)acetaMide
- CAS Number:1112982-76-9
- Molecular formula:C8H6BrN3OS
- Molecular Weight:272.12
![2-AMINO-5-BROMOTHIAZOLO[5,4-B]PYRIDINE](/CAS/GIF/934266-82-7.gif)
934266-82-7
62 suppliers
$23.00/250mg
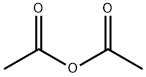
108-24-7
0 suppliers
$14.00/250ML
![N-(5-BroMothiazolo[5,4-b]pyridin-2-yl)acetaMide](/CAS/GIF/1112982-76-9.gif)
1112982-76-9
26 suppliers
$45.00/50mg
Yield:1112982-76-9 100%
Reaction Conditions:
with pyridine at 20;
Steps:
1
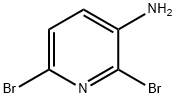
Example 1; Preparation of 7V-(5-Bromothiazolo[5,4-Z>]pyridin-2-yl)acetamide (1); 1A 1B Example 1[0376] To a 500 ml 3-neck under N2 was added KSCN (42.1 g, 433 mmol), followed by 225 mL of HOAc. It was cooled to -5 0C and 6-bromo-3-aminopyridine (15 g, 86.7 mmol) was added in portions. The mixture was further cooled to -15 0C, and a solution of Br2 (5.7 mL) in 12 mL of HOAc was added dropwise while keeping the bath temperature below 10 0C. It was then allowed to slowly warm up to room temperature and stirred overnight. The small amount of precipitate was filtered off. The mother liquor was cooled to 0 0C, and water was added (200 mL). It was stirred for 5 min, and the precipitate formed was collected and washed with cold MeOH/Et2O (1 :3, 50 mL, 2 times). The solid was dried under vacuum overnight to give 5-bromothiazolo[5,4-b]pyridin-2- amine the titled compound as a yellow solid (10.2 g, 51%). The solvent was stripped from the filtrate to half the volume, and the precipitation was collected, washed with cold MeOH-Et2O, and dried to give another 2 g of product with a combined yield of 12.2 g (61% yield). To the above obtained compound (11.3 g, 40 mmol) in pyridine (88 mL) was slowly added Ac2O (44 mL) at 0 0C. The mixture was then stirred at room temperature for 16 h. Solvent was removed, and the residue was subjected to vacuum for 20 h to give N- (5-bromothiazolo[5,4-b]pyridin-2-yl)acetamide as a light brown solid (13.3 g, 100%). [M+H] calc'd for C8H6BrN3OS, 272; found, 272.
References:
WO2010/8847,2010,A2 Location in patent:Page/Page column 123
39856-57-0
293 suppliers
$10.00/1g
13250-46-9
22 suppliers
$195.00/5ml
![N-(5-BroMothiazolo[5,4-b]pyridin-2-yl)acetaMide](/CAS/GIF/1112982-76-9.gif)
1112982-76-9
26 suppliers
$45.00/50mg

13250-46-9
22 suppliers
$195.00/5ml
169833-70-9
114 suppliers
$9.00/250mg
![N-(5-BroMothiazolo[5,4-b]pyridin-2-yl)acetaMide](/CAS/GIF/1112982-76-9.gif)
1112982-76-9
26 suppliers
$45.00/50mg