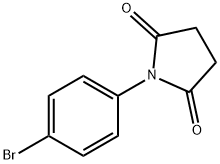
N-(4-BROMOPHENYL)SUCCINIMIDE synthesis
- Product Name:N-(4-BROMOPHENYL)SUCCINIMIDE
- CAS Number:41167-74-2
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
![4-[(4-bromophenyl)amino]-4-oxobutanoic acid](/CAS/GIF/25589-41-7.gif)
25589-41-7
16 suppliers
$28.60/50MG

41167-74-2
26 suppliers
$28.60/50mg
Yield:41167-74-2 91%
Reaction Conditions:
with ammonium peroxodisulfate in 1,4-dioxane;dimethyl sulfoxide at 100; for 6 h;
Steps:
General experimental procedure for the synthesis of imides:
General procedure: A solution of amine (1 equiv) and anhydride (1.1 equiv) in 1,4-dioxane was stirred at room temperature (or 100 °C if necessary) in a two neck round bottom flask equipped with a water condenser. As soon as all the amine converts to the corresponding amic acid (monitored by TLC), ammonium persulfate [(NH4)2S2O8] (2 equiv) and DMSO (2 equiv) were added and the reaction mixture was heated to 100 °C. Heating was continued at the same temperature until completion (3-10 h) of the reaction. After completion, the reaction mixture was filtered through a cotton plug and dioxane was removed under vacuo. The residue was dissolved in ethyl acetate and washed with dilute HCl, saturated aqueous NaHCO3 and brine. The organic layer was dried over anhydrous Na2SO4 and evaporated under vacuo to furnish the corresponding imides in good to excellent yields with more than 98% purity (GC, NMR).
References:
Garad, Dnyaneshwar N.;Tanpure, Subhash D.;Mhaske, Santosh B. [Beilstein Journal of Organic Chemistry,2015,vol. 11,p. 1008 - 1016] Location in patent:supporting information
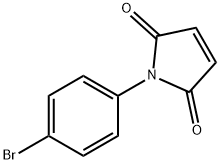
13380-67-1
99 suppliers
$18.00/1g

41167-74-2
26 suppliers
$28.60/50mg
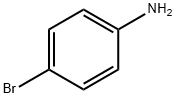
106-40-1
491 suppliers
$12.00/5g

41167-74-2
26 suppliers
$28.60/50mg
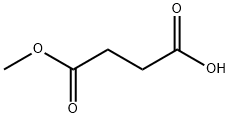
3878-55-5
250 suppliers
$9.00/1g

106-40-1
491 suppliers
$12.00/5g

41167-74-2
26 suppliers
$28.60/50mg
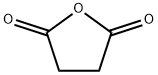
108-30-5
670 suppliers
$5.00/25g

106-40-1
491 suppliers
$12.00/5g

41167-74-2
26 suppliers
$28.60/50mg