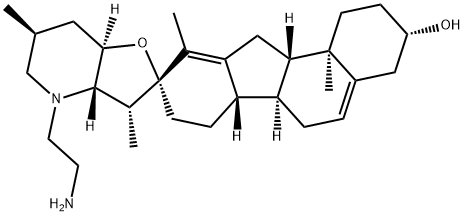
N-(2-AMINOETHYL) CYCLOPAMINE synthesis
- Product Name:N-(2-AMINOETHYL) CYCLOPAMINE
- CAS Number:334616-31-8
- Molecular formula:C29H46N2O2
- Molecular Weight:454.69
![Ethanone, 1-[(2'R,3S,3'R,3'aS,6'S,6aS,6bS,7'aR,11aS,11bR)-1,2,3,3',3'a,4,5',6,6',6a,6b,7,7',7'a,8,11,11a,11b-octadecahydro-3-hydroxy-3',6',10,11b-tetramethylspiro[9H-benzo[a]fluorene-9,2'(4'H)-furo[3,2-b]pyridin]-4'-yl]-2-amino-](/CAS/20210305/GIF/334616-30-7.gif)
334616-30-7
0 suppliers
inquiry

334616-31-8
12 suppliers
$600.60/1mg
Yield:334616-31-8 88%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran; for 3 h;Heating / reflux;
Steps:
Lithium aluminum hydride (939 μL of a 1M solution in THF, 939 μmoles) was added to a suspension of 16 (110 mg, 235 μmoles) in THF (6 mL). The reaction was refluxed for 3 h and then quenched with water (5 mL) and aqueous KOH (10 mL of a 10% solution). After extracting the mixture with chloroform (2 x 20 mL), the organic layer was dried over Na2SO4, filtered, and concentrated in vacuo. Purification by flash chromatography (SiO2, step-wise gradient from 20:1:0.1 to 20:2:0.1 chloroform/methanol/triethylamine) yielded the diamine as a colorless oil (94.4 mg, 208 μmoles, 88%). LRMS: (ES+) calcd for C29H46N2O2 (M + H): 455; found: 455. 1H NMR: spectrum is consistent with the predicted structure.
References:
EP1235851,2006,B1 Location in patent:Page/Page column 47