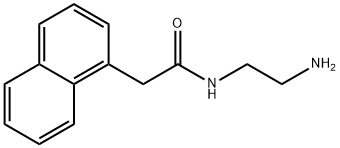
N-(2-AMINOETHYL)-2-(1-NAPHTHYL)ACETAMIDE synthesis
- Product Name:N-(2-AMINOETHYL)-2-(1-NAPHTHYL)ACETAMIDE
- CAS Number:36321-43-4
- Molecular formula:C14H16N2O
- Molecular Weight:228.29
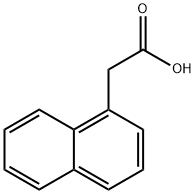
86-87-3
548 suppliers
$13.50/25G
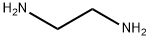
107-15-3
9 suppliers
$11.19/25ML

36321-43-4
72 suppliers
$185.00/100mg
Yield: 70%
Reaction Conditions:
Stage #1:naphth-1-yl acetic acid with 1-hydroxy-pyrrolidine-2,5-dione;diisopropyl-carbodiimide in chloroform at 20; for 1 h;
Stage #2:ethylenediamine in chloroform at 0 - 20;
Steps:
4.1.1 General procedure for synthesis of compound 6a-6n
General procedure: To a 250 mL round-bottomed flask add 1-Naphthylacetic acid (1b, 2.50g, 13.5mmol) or phenylacetic acid derivatives (1d-1f), equimolar N-hydroxysuccinimide (NHS) and about 30mL CHCl3, the resulting mixture was stirred at room temperature. Equimolar N,N′-diisopropylcarbodiimide (DIC) in about 10mL CHCl3 as coupling reagent was added to the above mixture with rapid stirring. After addition of DIC was completed, the reaction was stirred at room temperature for additional 1h. The product was filtrated, and the filtrate was diluted with CHCl3 to 150mL, then was added dropwise with vigorous stirring to 12 equiv ethylenediamine (9.76g, 162mmol) in 25mL CHCl3 at 0°C. The reaction continued at room temperature overnight. The solution was filtrated, and the filtrate was concentrated to 60 mL, washed four times with 10% sodium chloride solution and dried over anhydrous sodium sulfate. The solvents were removed in vacuo, flash chromatography of the residue on silica gel (CH2Cl2/CH3OH=8:1, v/v) gave pure product (2b, 2d-2f).
References:
Jin, Feng;Gao, Dan;Wu, Qin;Liu, Feng;Chen, Yuzong;Tan, Chunyan;Jiang, Yuyang [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 18,p. 5694 - 5706]
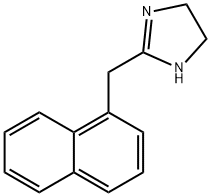
835-31-4
109 suppliers
inquiry

36321-43-4
72 suppliers
$185.00/100mg