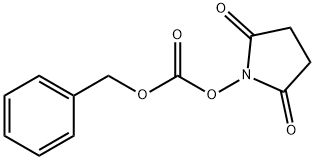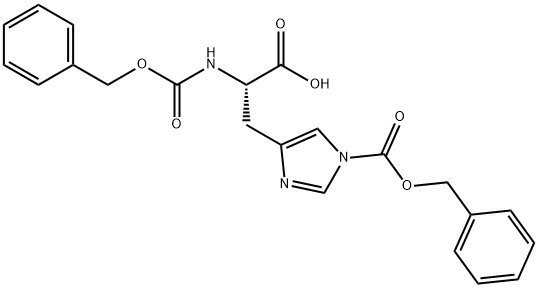
N,1-Bis-Cbz-L-histidine synthesis
- Product Name:N,1-Bis-Cbz-L-histidine
- CAS Number:35016-67-2
- Molecular formula:C22H21N3O6
- Molecular Weight:423.42
Yield:-
Reaction Conditions:
with N,N-didodecyl-N,N-dimethylammonium bromide;N-ethyl-N,N-diisopropylamine in dichloromethane at 5 - 20; for 8 h;Reagent/catalyst;
Steps:
1-5 Example 2:
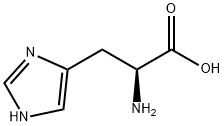
In a 5L three-necked flask, add 2L of dichloromethane, and then add 175g of L-histidine Add to it, the suspension will hardly heat up, and the temperature of the ice-salt bath will be cooled to 5°C, Add 438g of N,N-diisopropylethylamine to the system with a slight temperature rise, The reaction liquid becomes thinner, and then the catalyst is dodecyl dimethyl ammonium bromide 2g Add to the system, control the temperature at 5°C, add 647 g of N-benzyloxycarbonyloxysuccinimide to the system in batches, and the system generates heat. After adding N-benzyloxycarbonyloxysuccinimide, Increase the stirring speed, slowly warm up to room temperature and react for 8 hours. After the reaction is over, the system temperature is reduced to 10°C, and concentrated hydrochloric acid is used for about 400m l Adjust PH to 1, exothermic, let stand for half an hour, and separate liquids. The obtained organic phase was backwashed with 2L of water for liquid separation, The obtained organic phase was dried with anhydrous sodium sulfate, filtered with suction, A dichloromethane solution of intermediate bisCBZ-L-histidine is obtained.
References:
CN112898204,2021,A Location in patent:Paragraph 0047; 0055-0057; 0063-0065; 0071-0073; 0079-0081
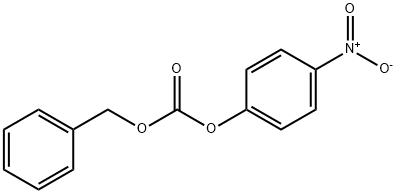
13795-24-9
85 suppliers
$5.00/100mg
71-00-1
732 suppliers
$7.00/5g
14997-58-1
200 suppliers
$11.00/1g

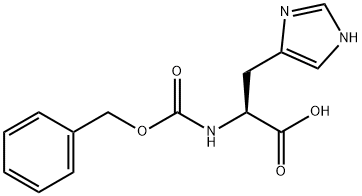
35016-67-2
15 suppliers
$35.00/1g
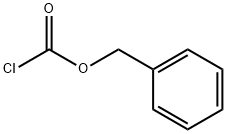
501-53-1
398 suppliers
$10.00/1g

71-00-1
732 suppliers
$7.00/5g

14997-58-1
200 suppliers
$11.00/1g

35016-67-2
15 suppliers
$35.00/1g
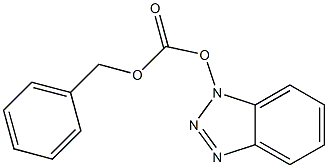
59577-41-2
1 suppliers
inquiry

71-00-1
732 suppliers
$7.00/5g

14997-58-1
200 suppliers
$11.00/1g

35016-67-2
15 suppliers
$35.00/1g