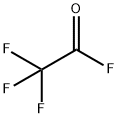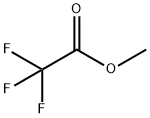
Methyl trifluoroacetate synthesis
- Product Name:Methyl trifluoroacetate
- CAS Number:431-47-0
- Molecular formula:C3H3F3O2
- Molecular Weight:128.05
Yield:431-47-0 93.8%
Reaction Conditions:
with potassium fluoride at 15; for 4 h;Large scale;
Steps:
4 Example 4
In a reaction kettle with a stirrer, add 40 kg of methanol.Slowly add 58 kg of potassium fluoride and mix the two together to obtain a mixture of ethanol and potassium fluoride.Transfer the mixture of ethanol and potassium fluoride to a closed reactor with a stirrer and start stirring.116 kg of trifluoroacetyl fluoride gas was passed into a mixture of ethanol and potassium fluoride for reaction,The reaction temperature is controlled at about 15 ° C, and the reaction time is controlled at 4 hours.After the reaction is completed, the reaction product is filtered to remove solids (KHF2), and the filtrate is transferred to a rectification column for rectification.120 kg of methyl trifluoroacetate were obtained (93.8% yield).KHF2 removed, which is a solid, neutralized with KOH after dissolving in water,All were converted to KF, which was dehydrated and dried to anhydrous potassium fluoride.
References:
Sanming Haisifu Chemical Co., Ltd.;Cao Wei;Lv Tao;Xie Weidong;Zhang Wei CN104370739, 2016, B Location in patent:Paragraph 0025-0026
123-76-2
485 suppliers
$5.00/25g
76-05-1
704 suppliers
$9.00/1g
110-15-6
975 suppliers
$5.00/10g

431-47-0
290 suppliers
$10.00/5g

123-76-2
485 suppliers
$5.00/25g

76-05-1
704 suppliers
$9.00/1g
64-18-6
1105 suppliers
$22.12/250ML

110-15-6
975 suppliers
$5.00/10g

431-47-0
290 suppliers
$10.00/5g
64-19-7
1576 suppliers
$10.00/25ML
503-66-2
213 suppliers
$28.00/1g
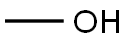
67-56-1
771 suppliers
$7.29/5ml-f
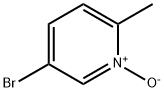
31181-64-3
91 suppliers
$60.00/100mg

900186-89-2
2 suppliers
inquiry

431-47-0
290 suppliers
$10.00/5g
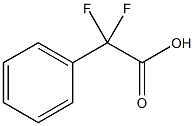
360-03-2
155 suppliers
$16.00/1g

431-47-0
290 suppliers
$10.00/5g