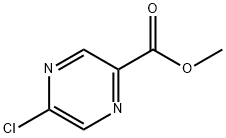
Methyl 5-chloropyrazine-2-carboxylate synthesis
- Product Name:Methyl 5-chloropyrazine-2-carboxylate
- CAS Number:33332-25-1
- Molecular formula:C6H5ClN2O2
- Molecular Weight:172.57
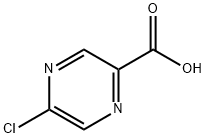
36070-80-1
275 suppliers
$9.00/1g
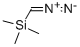
18107-18-1
215 suppliers
$33.00/5g

33332-25-1
243 suppliers
$8.00/1g
Yield:33332-25-1 100%
Reaction Conditions:
in methanol;diethyl ether for 0.5 h;
Steps:
(5-chloropyrazin-2-yl)methyl methanesulfonate; [00369] To a solution of 5-chloropyrazine-2-carboxylic acid (3.21 g, 20.3 mmol) in diethyl ether (20 mL) and methanol (20.0 mL) was added a 2M solution in diethyl ether of trimethylsilyldiazomethane (20.3 mL, 40.5 mmol). A vigorous bubbling was observed initially, and LCMS after 30 minutes indicated that the reaction was complete.Concentration of the reaction mixture afforded methyl 5-chloropyrazine-2-carboxylate (3.53 g, 20.5 mmol, 101% yield) as a tan solid. This material was shown to be >95% pure by NMR analysis and was used in the subsequent step without any purification. lH NMR (400 MHz, CDC13) δ (ppm): 9.09 (s, 1H), 8.70 (s, 1H), 4.04 (s, 3H).[00370] To a 0 °C solution of methyl 5-chloropyrazine-2-carboxylate (3.50 g, 20.3 mmol) in tetrahydrofuran (101 mL) was added a 1M solution in tetrahydrofuran of diisobutylaluminum hydride (42.6 mL, 42.6 mmol). The reaction was stirred at 0 °C for 2 hours, after which it was quenched by the addition of methanol (2 mL). To this mixture was added saturated sodium-potassium tartrate solution, and the resulting reaction mixture was extracted with ethyl acetate (3 x 100 mL), dried (sodium sulfate), filtered and concentrated to brown residue. Purification was achieved by column chromatography on silica gel (Luknova 120 g, 20 mL/min) using 30 to 100% ethyl acetate in hexanes over 60 minutes to afford (5- chloropyrazin-2-yl)methanol (1.45 g, 10.0 mmol, 50 % yield) as a tan solid. NMR (400 MHz, CDC13) a (ppm): 8.56 (s, 1H), 8.45 (s, 1H), 4.84 (s, 3H), 2.79 (br. s, 1H).[00371] To a 0 °C solution of (5-chloropyrazin-2-yl)methanol (648 mg, 4.48 mmol) in dichloromethane (12 mL) was added triethylamine (1.87 mL, 13.5 mmol) followed by dropwise addition of methanesulfonyl chloride (0.699 mL, 8.97 mmol). After 20 minutes, analysis by LCMS indicated the complete conversion to the mesylate product. The reaction mixture was concentrated to afford (5-chloropyrazin-2-yl)methyl methanesulfonate (787 mg, 3.53 mmol, 79% yield) as an oil. The material was used crude in the next step without further purification.
References:
IRONWOOD PHARMACEUTICALS, INC.;HUDSON, Colleen;BARDEN, Timothy, C.;JIA, James;MERMERIAN, Ara;PENG, Bo;YANG, Jane;YU, Xiang, Y.;SPROTT, Kevin WO2012/88469, 2012, A1 Location in patent:Page/Page column 151-152
13924-95-3
134 suppliers
$10.00/250mg

33332-25-1
243 suppliers
$8.00/1g
67-56-1
774 suppliers
$7.29/5ml-f

36070-80-1
275 suppliers
$9.00/1g

33332-25-1
243 suppliers
$8.00/1g
67-56-1
774 suppliers
$7.29/5ml-f

33332-25-1
243 suppliers
$8.00/1g
67-56-1
774 suppliers
$7.29/5ml-f
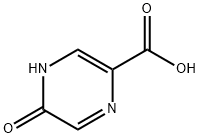
34604-60-9
207 suppliers
$15.00/1g

33332-25-1
243 suppliers
$8.00/1g