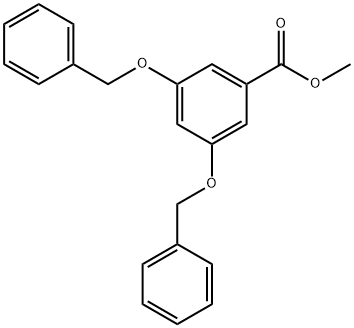
METHYL 3,5-DIBENZYLOXYBENZOATE synthesis
- Product Name:METHYL 3,5-DIBENZYLOXYBENZOATE
- CAS Number:58605-10-0
- Molecular formula:C22H20O4
- Molecular Weight:348.39
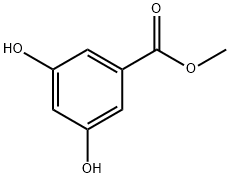
2150-44-9
269 suppliers
$5.00/5g
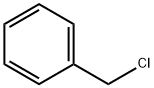
100-44-7
657 suppliers
$13.50/250G

58605-10-0
111 suppliers
$19.00/1g
Yield:58605-10-0 100%
Reaction Conditions:
with iodide;potassium carbonate in acetone for 7 h;Reflux;
Steps:
3,4-bis(Benzyloxy)benzoic acid (45) [51];
General procedure: Methyl 3,4-dihydroxybenzoate (2.5 g, 15 mmol) and K2CO3(8.2 g, 60 mmol) and KI (2.0 g, 12 mmol) in 100 mL of acetone was stirred at rt. The reaction suspensionwas slowly added BnCl (4.2 mL, 36 mmol) and refluxed for 7 h. TLC indicated full conversion of thestart material, added MeOH and stirred 1 h. The reaction mixture was filtered by celite and filtrate wasevaporated under reduced pressure. The residue was purified by recrystallization with hexane, and themother liquid was purified by C.C (Hex/EtOAc = 100/1-4/1) to aord methyl ester of 45 (total 5.0 g,97%) as a white solid. Further on, methyl ester of 45 (3.0 g, 9.0 mmol), KOH (5.0 g, 90 mmol) in90 mL of 1,4-dioxane and 30 mL of MeOH was stirred at 85 °C for 2 h. TLC indicated full conversionof the start material, the reaction mixture was cooled to 0 C and slowly added 6 M HCl until pH = 1.The precipitating muddy suspension was filtrated, and the white residue was washed by water andMeOH until pH = 7. The white solid was dried in vacuo, purified by recrystallizing with MeOHto obtain desired compound 45 (2.4 g, 79%) as colorless needles.
References:
MacHida, Shota;Mukai, Saki;Kono, Rina;Funato, Megumi;Saito, Hiroaki;Uchiyama, Taketo [Molecules,2019,vol. 24,# 23,art. no. 4340]

2150-44-9
269 suppliers
$5.00/5g
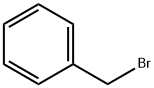
100-39-0
436 suppliers
$10.00/10 g

58605-10-0
111 suppliers
$19.00/1g

2150-44-9
269 suppliers
$5.00/5g

100-39-0
436 suppliers
$10.00/10 g

58605-10-0
111 suppliers
$19.00/1g
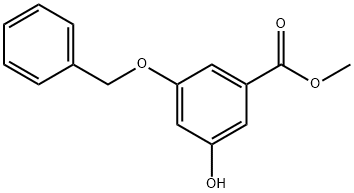
54915-31-0
82 suppliers
$23.00/250mg
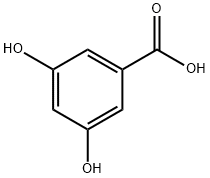
99-10-5
541 suppliers
$5.00/5g

58605-10-0
111 suppliers
$19.00/1g