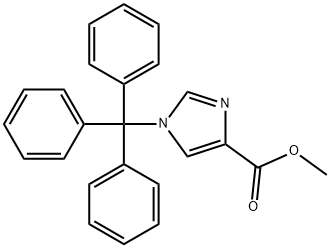
Methyl 1-trityl-1H-iMidazole-4-carboxylate synthesis
- Product Name:Methyl 1-trityl-1H-iMidazole-4-carboxylate
- CAS Number:76074-88-9
- Molecular formula:C24H20N2O2
- Molecular Weight:368.43
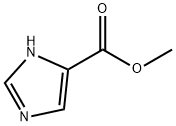
17325-26-7
234 suppliers
$13.00/250mg
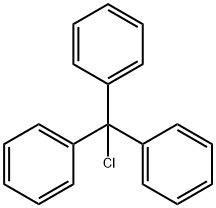
76-83-5
708 suppliers
$6.00/25g

76074-88-9
23 suppliers
$360.80/1g
Yield:76074-88-9 100%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide at 20; for 2 h;
Steps:
2 Example 2: Preparation of methyl 1-triphenylmethyl-1H-imidazole-4-carboxylate
Methyl 1H-imidazole-4-carboxylate (96.5 g, 765.1 mmol) was dissolved in 1.8 LIn N,N-dimethylformamide (DMF), then triphenylchloromethane (213.3 g, 765.1 mmol) was added.Finally, triethylamine (85.2 g, 841.6 mmol) was added.After the addition, the nitrogen was replaced three times, and the reaction was carried out at room temperature for about 2 hours.The TLC detects the completion of the reaction and terminates the reaction. Post processing:The reaction system was slowly poured into 6 L of water, stirred and lysed for 1.0 h, filtered, and the filter cake was washed with 500 ml of water.Drying at 50 ° C for 4 h gave 282.0 g of an off-white solid in a yield of 100.0%.
References:
CN108314654,2018,A Location in patent:Paragraph 0090-0092
596-43-0
124 suppliers
$16.00/5g

17325-26-7
234 suppliers
$13.00/250mg

76074-88-9
23 suppliers
$360.80/1g

76-83-5
708 suppliers
$6.00/25g

17325-26-7
234 suppliers
$13.00/250mg

76074-88-9
23 suppliers
$360.80/1g