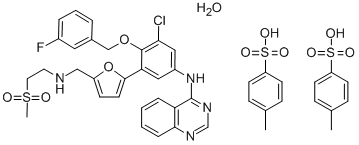
Lapatinib ditosylate monohydrate synthesis
- Product Name:Lapatinib ditosylate monohydrate
- CAS Number:388082-78-8
- Molecular formula:C41H40ClFN4O11S3
- Molecular Weight:925.46
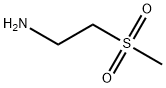
49773-20-8
59 suppliers
$41.00/250mg
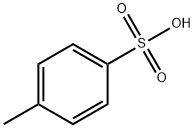
104-15-4
748 suppliers
$133.00/500g
![5-[4-((3-Chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-furaldehyde](/CAS/GIF/231278-84-5.gif)
231278-84-5
288 suppliers
$8.00/250mg

388082-78-8
349 suppliers
$6.00/50mg
Yield:388082-78-8 93%
Reaction Conditions:
Stage #1: 2-Methanesulfonyl-ethylamine;5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)quinazolin-6-yl)furan-2-carbaldehyde in methanol;dichloromethane at 20;
Stage #2: with hydrogen in methanol;dichloromethane;Inert atmosphere;
Stage #3: toluene-4-sulfonic acid in methanol;dichloromethane;
Steps:
7
Example 7:Preparation of A/-(3-chloro-4-{[(3-fluorophenyl)methyl]oxy}phenyl)-6-[5-({[2- (methylsulfonyl)ethyl]amino}methyl)-2-furanyl]-4-quinazolinamine bis 4- methylbenzenesulfonate.The suspension of 5-[4-((3-chloro-4-((3-fluorobenzyl)oxy)phenyl)amino)quinazolin-6-yl]-2-furaldehyde (5 g, 10.6 mmoL) in dichloromethane (50ml_) was charged with a solution of 2-aminoethylmethylsulfone (3.2 g, 1 1.7mmoL) in methanol (25ml_) slowly under constant stirring at room temperature. The reaction mixture was stirred for 2-4 hours until reaction completion. The reaction mixture was charged with 5% Pd-C (750mg) and stirred under hydrogen atmosphere using balloon pressure for 12-16 hours until reaction completion. The obtained mixture was filtered through Celite pad and rinsed with methanol (5 mL) and dichloromethane (15 mL) mixture. The filtrate was distilled to low volume and the solution was charged with dichloromethane (25 mL) followed by addition of the solution of p-toluenesulfonic acid monohydrate (4.4 g, 23.3 mmoL) in methanol (10 mL). The yellow solid which precipitated out was filtered after 2- 8 hours and washed with 1 :1 mixture of methanol and dichloromethane (20 mL). The solid obtained was dried under vacuum at 40-45°C to provide Lapatinib (1 1.4 g, Yield = 93%, HPLC purity >99%).
References:
WO2012/83440,2012,A1 Location in patent:Page/Page column 13-14

1227853-06-6
3 suppliers
inquiry

104-15-4
748 suppliers
$133.00/500g

388082-78-8
349 suppliers
$6.00/50mg
231277-92-2
493 suppliers
$20.00/50mg

388082-78-8
349 suppliers
$6.00/50mg
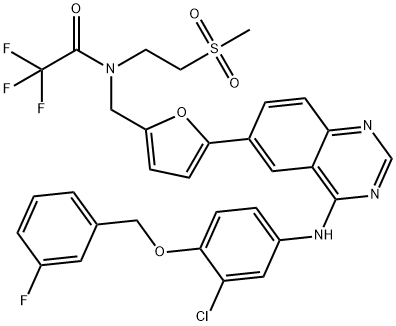
1618648-59-1
2 suppliers
inquiry

104-15-4
748 suppliers
$133.00/500g

388082-78-8
349 suppliers
$6.00/50mg
![N-[3-Chloro-4-(3-fluorobenzyloxy)phenyl]-6-iodoquinazolin-4-amine](/CAS/GIF/231278-20-9.gif)
231278-20-9
301 suppliers
$10.00/250mg

388082-78-8
349 suppliers
$6.00/50mg