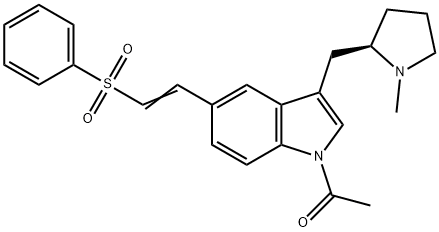
(R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole synthesis
- Product Name: (R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole
- CAS Number:180637-88-1
- Molecular formula:C24H26N2O3S
- Molecular Weight:422.54
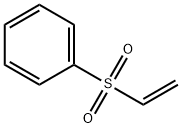
5535-48-8
259 suppliers
$12.00/1g
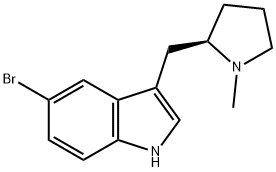
143322-57-0
169 suppliers
$18.00/1g
![(R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole](/CAS/20200611/GIF/180637-88-1.gif)
180637-88-1
6 suppliers
inquiry
Yield:180637-88-1 83%
Reaction Conditions:
with triethylamine;palladium diacetate;tris-(o-tolyl)phosphine in acetonitrile; for 7.5 h;Heating / reflux;Industry scale;
Steps:
1; 1.b Scheme 1
(b) A mixture of acetonitrile (375 kg), palladium acetate (12.5 kg) and tri-o-tolylphosphine (60 kg) was stirred for 1 hour. Phenyl vinyl sulphone (160 kg), triethylamine (92 kg) and finally the solution prepared in part (a) were added and the mixture was heated to reflux for 7.5 hours. The reaction mixture was cooled and a solution of 190 kg concentrated hydrochloric acid in 1200 kg water was added over 4 hours. The resulting mixture was filtered to remove spent catalyst and a further 3000 kg of water and 300 kg of 50% w/w aqueous sodium hydroxide solution were added to the filtrate to precipitate the product. The resulting suspension was filtered and washed with water (500 kg) to yield crude (R)-1-acetyl-5-(2-phenylsulphonylethenyl)-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole) as a dark brown, wet, crystalline solid (535 kg wet, equivalent of 338 kg dry). This crude product was added to 530 kg of acetone and the mixture was heated to 60° C. On reaching this temperature 814 kg of water was added over 2 hours whilst simultaneously cooling the mixture back to ambient temperature. The batch was then granulated for 2 hours and filtered to yield the purified product (350 kg wet, equivalent of 280 kg dry, 83%).
References:
US2005/20663,2005,A1 Location in patent:Page 2-3
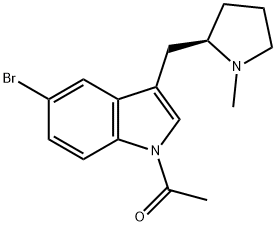
205369-12-6
34 suppliers
$59.00/2mg

5535-48-8
259 suppliers
$12.00/1g
![(R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole](/CAS/20200611/GIF/180637-88-1.gif)
180637-88-1
6 suppliers
inquiry

5535-48-8
259 suppliers
$12.00/1g
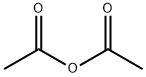
108-24-7
0 suppliers
$14.00/250ML

143322-57-0
169 suppliers
$18.00/1g
![(R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole](/CAS/20200611/GIF/180637-88-1.gif)
180637-88-1
6 suppliers
inquiry
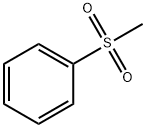
3112-85-4
210 suppliers
$15.00/5g

5535-48-8
259 suppliers
$12.00/1g

108-24-7
0 suppliers
$14.00/250ML

143322-57-0
169 suppliers
$18.00/1g
![(R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole](/CAS/20200611/GIF/180637-88-1.gif)
180637-88-1
6 suppliers
inquiry

143322-57-0
169 suppliers
$18.00/1g
![(R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole](/CAS/20200611/GIF/180637-88-1.gif)
180637-88-1
6 suppliers
inquiry