fluromidine synthesis
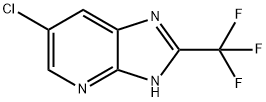
- Product Name:fluromidine
- CAS Number:13577-71-4
- Molecular formula:C7H3ClF3N3
- Molecular Weight:221.57
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

13577-71-4
23 suppliers
inquiry
Yield:-
Reaction Conditions:
in N-methyl-acetamide;sodium hydroxide;thionyl chloride;water
Steps:
1 PREPARATION OF 6-CHLORO-2-(TRIFLUOROMETHYL)-1H-IMIDAZO[4,5-b]PYRIDINE
EXAMPLE 1 PREPARATION OF 6-CHLORO-2-(TRIFLUOROMETHYL)-1H-IMIDAZO[4,5-b]PYRIDINE Dimethylformamide (2 milliliters) was added to 4 grams of 1-hydroxy-2-(trifluoromethyl)-1H-imidazo[4,5-b]pyridine in 10 milliliters of thionyl chloride. The resulting reaction mixture was heated on a steam bath overnight. Solvent was removed and the residue shaken with 50 milliliters of water and filtered. The residue was then taken up in sodium hydroxide solution, filtered and acidified to pH 3, shaken with three 150-milliliter portions of diethyl ether, and dried over magnesium sulfate. Solvent was evaporated, yielding the desired 6-chloro-2-(trifluoromethyl)-1H-imidazo[4,5-b]pyridine, m.p., 290°-2° C., with sublimation from 260° C.
References:
Eli Lilly and Company US4031107, 1977, A
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

13577-71-4
23 suppliers
inquiry