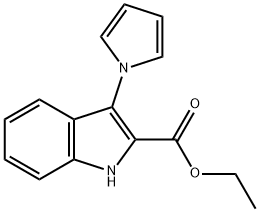
ETHYL 3-(1H-PYRROL-1-YL)-1H-INDOLE-2-CARBOXYLATE synthesis
- Product Name:ETHYL 3-(1H-PYRROL-1-YL)-1H-INDOLE-2-CARBOXYLATE
- CAS Number:344277-22-1
- Molecular formula:C15H14N2O2
- Molecular Weight:254.28
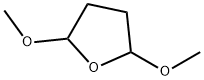
696-59-3
393 suppliers
$7.00/25g
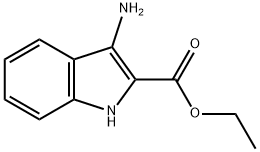
87223-77-6
68 suppliers
$87.00/100mg

344277-22-1
11 suppliers
$442.00/1g
Yield:344277-22-1 91%
Reaction Conditions:
Stage #1: cis,trans-2,5-dimethoxytetrahydrofuranwith 4-chlorpyridine hydrochloride in 1,4-dioxane at 20; for 0.25 h;Clauson-Kaas reaction;
Stage #2: ethyl 3-amino-1H-indole-2-carboxylate in 1,4-dioxane;Reflux;Clauson-Kaas reaction;
Steps:
4.3. General method for the synthesis of substituted ethyl 3-(1H-pyrrol-1-yl)-1H-indole-2-carboxylates (9a-g)
General procedure: A solution of 2,5-dimethoxytetrahydrofuran (0.14 mL, 1.1 mmol) in dioxan (10 mL), was stirred for 15 min with 4-chloropyridine hydrochloride (165 mg, 1.1 mmol). The appropriate 3-aminoindole (8a-g) (1.1 mmol) was added and the reaction mixture was refluxed for 1-4 h and, then filtered through a small pad of Celite. The solvent was evaporated to give a brown residue that was dissolved in dichloromethane (100 mL). The solution was washed with a 1 M aqueous hydrochloric acid solution. The organic layer was dried (Na2SO4) and evaporated to give a crude solid, which was purified by chromatography column using dichloromethane as eluant, to give 3-(1H-pyrrol-1-yl)-indoles (9a-g).
References:
Diana, Patrizia;Stagno, Antonina;Barraja, Paola;Montalbano, Alessandra;Carbone, Anna;Parrino, Barbara;Cirrincione, Girolamo [Tetrahedron,2011,vol. 67,# 19,p. 3374 - 3379] Location in patent:experimental part