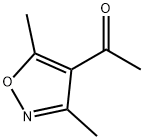
Ethanone, 1-(3,5-dimethyl-4-isoxazolyl)- (9CI) synthesis
- Product Name:Ethanone, 1-(3,5-dimethyl-4-isoxazolyl)- (9CI)
- CAS Number:35166-20-2
- Molecular formula:C7H9NO2
- Molecular Weight:139.15
1223452-45-6
11 suppliers
$90.00/100mg
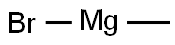
75-16-1
270 suppliers
$12.00/10ml

35166-20-2
22 suppliers
$175.00/100mg
Yield:35166-20-2 420 mg
Reaction Conditions:
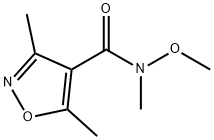
Stage #1: N-methoxy-N-methyl-3,5-dimethylisoxazole-4-carboxamide;methylmagnesium bromide in tetrahydrofuran at 0 - 20; for 4 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 20; for 1 h;
Steps:
4.2 1-(3,5-dimethylisoxazol-4-yl)ethanone
A solution of 580 mg (3.15 mmol) of N-methoxy-N-methyl-3,5-dimethylisoxazole-4-carboxamide in 20 mL of THF is cooled to 0°C. A solution of 1.57 mL (4.72 mmol) of 3 M methylmagnesium bromide in ether is added. After stirring for 4 hours at room temperature, the reaction medium is taken up in 10 mL of 1 N HCl and stirred for a further 1 hour at room temperature. The mixture is then basified with K2CO3 and extracted with ethyl acetate. The organic phase is dried over magnesium sulfate and evaporated to dryness to give 420 mg of 1-(3,5-dimethylisoxazol-4-yl)ethanone, corresponding to the following characteristics: LC/MS (method G): [M+H]+: m/z 140 tr (min) = 1.06. 1H NMR spectrum (300 MHz, δ in ppm, CDCl3): 2.48 (s, 6H), 2.70 (s, 3H).
References:
WO2013/190123,2013,A1 Location in patent:Page/Page column 48; 49
59402-44-7
1 suppliers
inquiry

35166-20-2
22 suppliers
$175.00/100mg
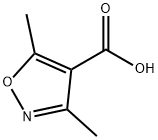
2510-36-3
195 suppliers
$5.00/1g

35166-20-2
22 suppliers
$175.00/100mg
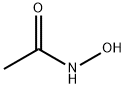
546-88-3
477 suppliers
$5.00/100mg
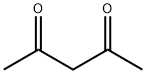
123-54-6
576 suppliers
$10.00/25ml

35166-20-2
22 suppliers
$175.00/100mg
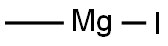
917-64-6
149 suppliers
$45.00/10ml
31301-46-9
30 suppliers
inquiry

35166-20-2
22 suppliers
$175.00/100mg