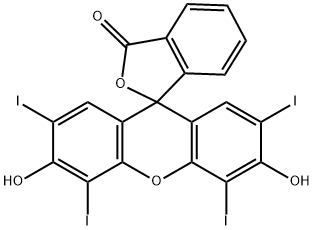
ERYTHROSIN B synthesis
- Product Name:ERYTHROSIN B
- CAS Number:15905-32-5
- Molecular formula:C20H8I4O5
- Molecular Weight:835.89
Yield:56254-06-9 70% ,15905-32-5 30%
Reaction Conditions:
with Oxone;sodium iodide at 200; for 12 h;
Steps:
3
EXAMPLE 3 Iodination Reaction of Fluorescein A mixture of the lactonic form of fluorescein (1 equivalent), 16 equivalents of NaI, and 16 equivalents of Oxone (2 KHSO5.KHSO4.K2SO4), is crushed in an agate mortar until a homogeneous and finely ground mixture is obtained. The solid mixture is placed in an oven at 200° C. for 12 hours. The iodination reaction leads to the formation of two products, a main one, 2',4',5'-triiodofluorescein (3I-F, about 70%) and a secondary one, tetraiododerivative (about 30%) which partly decomposes in the work-up of the mixture. Also in this case there is total consumption of the Fluorescein and a substantially quantitative overall yield. The product is characterized by Mass Spectrometry ESI and spectroscopic techniques (1H-NMR and 13C-NMR). In particular, 3I-F: ESI MS (-): 709.0 M-H-; 1H NMR (CH3OD); 6.75 ppm (d, J=8.0 Hz 7' H); 6.79 ppm (d, J=8.0 Hz 8' H); 7.34 (d, J=8.0 Hz 3H); 7.35 (s, 1' H) 7.78 (m, 4H and 5H) 8.15 (dd, J=8.0; 1.2 Hz 6H).
References:
US2008/61289,2008,A1 Location in patent:Page/Page column 7
2321-07-5
375 suppliers
$6.00/25g

15905-32-5
103 suppliers
$17.46/5G

64-17-5
739 suppliers
$10.00/50g

2321-07-5
375 suppliers
$6.00/25g

15905-32-5
103 suppliers
$17.46/5G
![Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 3',6'-dihydroxy-2',4',5'-triiodo-](/CAS/20210305/GIF/56254-06-9.gif)