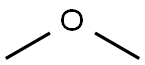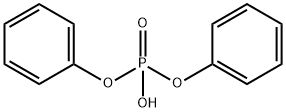
Diphenyl phosphate synthesis
- Product Name:Diphenyl phosphate
- CAS Number:838-85-7
- Molecular formula:C12H11O4P
- Molecular Weight:250.19
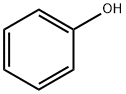
108-95-2
761 suppliers
$14.00/25g

838-85-7
278 suppliers
$9.00/1g
Yield:838-85-7 71%
Reaction Conditions:
with 1-Butylimidazole;phosphoric acid;triethylamine in N,N-dimethyl-formamide at 230; for 24 h;Inert atmosphere;Temperature;Reagent/catalyst;
Steps:
General procedure for the synthesis of diaryl phosphates
General procedure: Phosphoric acid 85% (461.2 mg, 4.0 mmol), phenol (3.76 g, 40 mmol), triethylamine (1.21 g, 12 mmol), 1-butylimidazole (149.0 mg, 1.2 mmol) and DMF (5 mL) were placed in the reaction apparatus consisted of a 50 mL three-necked flask with a tube through which the boiling solvent and water vapor flowed and a tube through which the solvent was refluxed through the dehydrating agent. (See Fig. S1) PAdeCS, 2.0 gram as a drying agent is put under the condenser. The reaction was heated at 230 °C for 24 h and after this time, the resulting mixture was run through cation exchange resin (eluent: MeOH) to remove amines. After evaporation of eluent, the mixture was distilled at low pressure to remove phenol and DMF. The desired diphenyl hydrogen phosphate is obtained by gel permeation chromatography GPC (eluent: CHCl3), 71% (619.6 mg, 2.84 mmol).
References:
Tran, Cong Chi;Asao, Kazuya;Sasaki, Takeshi;Hayakawa, Yasuyuki;Kawaguchi, Shin-ichi [Tetrahedron Letters,2022,vol. 96,art. no. 153726] Location in patent:supporting information
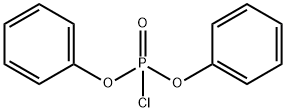
2524-64-3
392 suppliers
$14.00/5g

838-85-7
278 suppliers
$9.00/1g
![1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 3-[(diphenoxyphosphinyl)oxy]-4-methyl-7-oxo-6-[(1R)-1-[(trimethylsilyl)oxy]ethyl]-, (2,2-dimethyl-1-oxopropoxy)methyl ester, (4R,5R,6S)-](/CAS/20210305/GIF/692779-24-1.gif)
692779-24-1
0 suppliers
inquiry
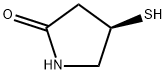
157429-42-0
45 suppliers
$180.00/250MG

838-85-7
278 suppliers
$9.00/1g
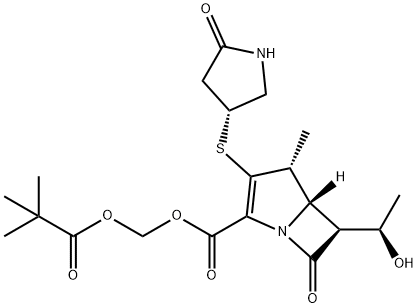
157542-49-9
3 suppliers
inquiry
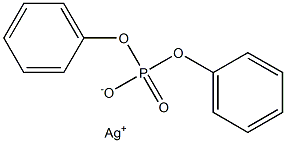
22350-95-4
1 suppliers
inquiry

838-85-7
278 suppliers
$9.00/1g