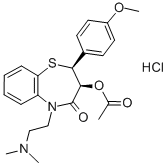
Diltiazem hydrochloride synthesis
- Product Name:Diltiazem hydrochloride
- CAS Number:33286-22-5
- Molecular formula:C22H27ClN2O4S
- Molecular Weight:450.98
42399-40-6
70 suppliers
$50.00/2mg
108-24-7
0 suppliers
$14.00/250ML

33286-22-5
521 suppliers
$16.00/250mg
Yield:33286-22-5 92%
Reaction Conditions:
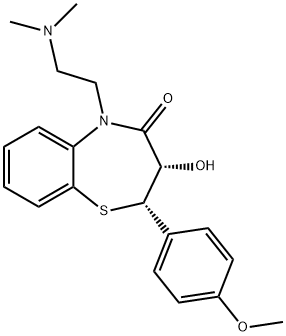
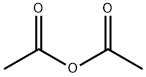
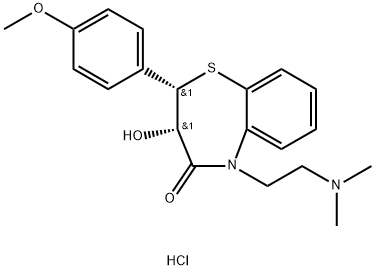
Stage #1: O-desacetyldiltiazem;acetic anhydridewith dmap;triethylamine in dichloromethane; for 3 h;Heating / reflux;
Stage #2: with hydrogenchloride in methanol; pH=2;
Steps:
2.d Preparation of Cis-(+)-3-(Acetyloxy)-2,3-dihydo-5-[2-(dimethylamino)-ethyl]-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one Hydrochloride (Diltiazem Hydrochloride)
The N-alkylated lactam (0.317 mmol), Ac2O (1 mmol), Et3N (2 mmol) and DMAP (0.03 mmol) taken in CH2Cl2 (5 mL) were heated at reflux under N2 for 3 h. The mixture was poured into ice water and brine was added. The organic layer was separated and the aqueous layer was extracted with CH2Cl2 (10 mL). The combined organic layers were washed with 5% NH4OH (5 mL) solution, dried (Na2SO4) and evaporated. The residue was dissolved in MeOH (2 mL) and treated with anhydrous HCl gas till pH was 2. Ether (3 mL) was added to the resulting solution. The precipitated solids were collected by filtration and washed with 10% MeOH-ether to afford diltiazem hydrochloride (92% yield).
References:
WO2004/58734,2004,A1 Location in patent:Page 7-8
75472-91-2
38 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML

33286-22-5
521 suppliers
$16.00/250mg
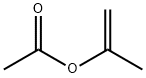
108-22-5
264 suppliers
$20.00/25mL

75472-91-2
38 suppliers
inquiry

33286-22-5
521 suppliers
$16.00/250mg
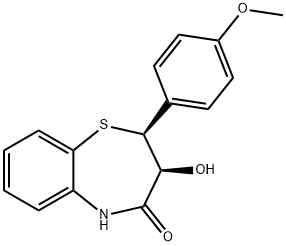
42399-49-5
184 suppliers
$6.00/5g

33286-22-5
521 suppliers
$16.00/250mg
![Benzenepropanoic acid, β-[(2-aminophenyl)thio]-α-hydroxy-4-methoxy-, 2-phenylcyclohexyl ester, hydrochloride, [1R-[1α(αS*,βS*),2β]]- (9CI)](/CAS/20211123/GIF/138382-02-2.gif)
138382-02-2
0 suppliers
inquiry

33286-22-5
521 suppliers
$16.00/250mg