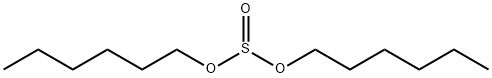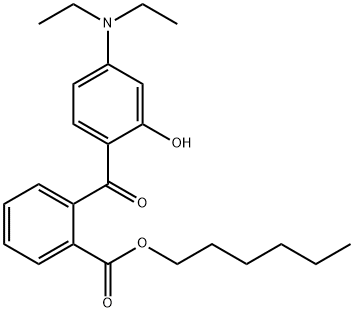
Diethylamino hydroxybenzoyl hexyl benzoate synthesis
- Product Name:Diethylamino hydroxybenzoyl hexyl benzoate
- CAS Number:302776-68-7
- Molecular formula:C24H31NO4
- Molecular Weight:397.51

Expose 3 mL of a 0.018 mg/mL solution of 3-diethylaminophenyl 2-hexanoxycarbonylbenzoate in methanol to UV-B radiation in a Luzchem LZC-4 photoreactor equipped with 16 broad band UV-B lamps (LES-UVB-01) at 35 °C to obtain Diethylamino hydroxybenzoyl hexyl benzoate.
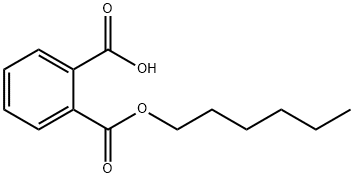
24539-57-9
49 suppliers
$115.00/1g
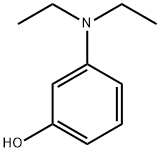
91-68-9
293 suppliers
$9.00/25g

302776-68-7
278 suppliers
$11.00/5g
Yield:302776-68-7 95%
Reaction Conditions:
with zinc(II) chloride;trichlorophosphate in dimethyl sulfoxide at 20 - 50; for 5 h;Solvent;Temperature;
Steps:
1.2; 1.3; 2.2; 2.3; 3.2; 3.3 (2) Acylation reaction:
Put 130g of 3-hydroxy-N,N-diethylaniline into the reactor,520g of monohexyl phthalate, 130g of anhydrous zinc chloride,130g phosphorus oxychloride and 1170g dimethyl sulfoxide, start stirring, stir and dissolve at room temperature,Raise the temperature to 50°C, keep the temperature for 5h,The hydrogen chloride produced during the reaction is absorbed by an absorption tower. After the reaction is completed, the solvent is recovered under reduced pressure.Then cool to room temperature; then slowly add 1800mL water into the reaction flask,While adding water, stir until the residue is dissolved, and then extract and separate with 570mL ethyl acetate.Extract three times, combine the organic phases,And add 26g of activated carbon to the organic phase to reflux for 30min, filter to remove the activated carbon,Recover ethyl acetate by distillation under reduced pressure to obtain 305g of ultraviolet absorber UVA Plus;(3) Refined crystals:Add 1000mL methanol to the ultraviolet absorber UVA Plus synthesized in the previous step,Heat to 60°C, stir to dissolve into a clear solution,Then slowly cool down to 0-2°C and stir to crystallize for 4 hours.During this period, white granular powder gradually separated out, filtered by suction,Obtained 298 g of ultraviolet absorber UVA Plus white powder crystal product, with a yield of 95%.The obtained UV absorber UVA Plus product is tested by liquid chromatography,The purity is 99.92%.
References:
CN111499529,2020,A Location in patent:Paragraph 0026; 0033; 0036-0041; 0044-0046; 0049-0050

544-10-5
239 suppliers
$13.00/5g
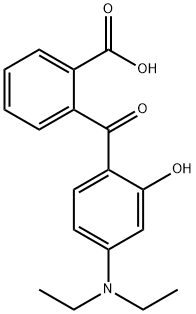
5809-23-4
193 suppliers
$5.00/1g

302776-68-7
278 suppliers
$11.00/5g

111-25-1
324 suppliers
$10.00/5g

5809-23-4
193 suppliers
$5.00/1g

302776-68-7
278 suppliers
$11.00/5g