CHEMBRDG-BB 4303055 synthesis
- Product Name:CHEMBRDG-BB 4303055
- CAS Number:114590-73-7
- Molecular formula:C11H14O2
- Molecular Weight:178.23
Yield:114590-73-7 94%
Reaction Conditions:
with potassium carbonate in acetone at 70;
Steps:
135.135-a.135-a-1
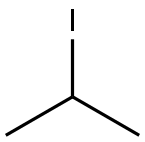
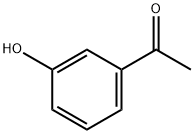
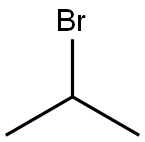
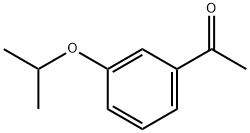
Example 135; Preparation of 3-((4-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-2-propylphenoxy)pyridin-2-yl)methyl)-5-(3-(1-methylethoxy)phenyl)-5-methylimidazolidine-2,4-dione; 135-a) Preparation of 5-[3-(1-methylethoxy)phenyl]-5-methylimidazolidine-2,4-dione; 135-a-1) Preparation of 1-(3-(1-methylethoxy)phenyl)ethanone; 1-(3-Hydroxyphenyl)ethanone (1.36 g, 10 mmol) was dissolved in acetone (50 mL), and the resultant mixture was sequentially added with potassium carbonate (2.76 g, 20 mmol) and 1-methylethyl iodide (1.5 mL, 15 mmol), and stirred at 70° C. overnight. The reaction solution was filtered, washed with acetone, and concentrated in vacuo. The resultant residue was added with water and ethyl acetate and extracted with ethyl acetate. The organic layer was washed with 1N aqueous solution of sodium hydroxide and brine, dried using anhydrous sodium sulfate, and concentrated in vacuo. 1-(3-(1-methylethoxy)phenyl)ethanone (1.67 g, yield 94%) was obtained as a yellow oil.1H-NMR (CDCl3) δ: 1.35 (6H, d, J=6.0 Hz), 2.55 (3H, s), 4.63 (1H, sept, J=6.0 Hz), 7.09 (1H, dd, J=2.4, 8.3 Hz), 7.35 (1H, t, J=8.0 Hz), 7.48-7.52 (2H, m).
References:
US2010/48610,2010,A1 Location in patent:Page/Page column 79