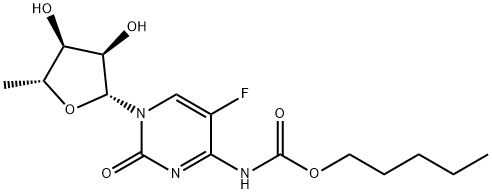
Capecitabine synthesis
- Product Name:Capecitabine
- CAS Number:154361-50-9
- Molecular formula:C15H22FN3O6
- Molecular Weight:359.35
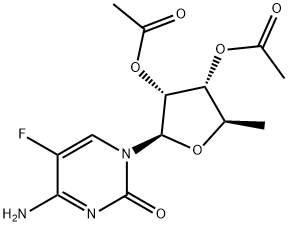
![5'-deoxy-5-fluore-N-[(pentoyloxy)carbonyl]cytidine 2',3'-diacetate](/CAS/GIF/162204-20-8.gif)
162204-20-8
289 suppliers
$5.00/250mg

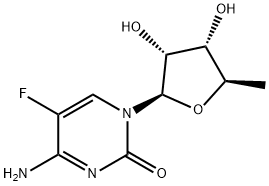
154361-50-9
819 suppliers
$5.00/1g
Yield:154361-50-9 98%
Reaction Conditions:
Stage #1: 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidinewith water;sodium hydroxide in methanol at -15;
Stage #2: with hydrogenchloride in methanol;water at -15; pH=5;
Steps:
5'-Deoxy-5-fluoro-N4-(n-pentyloxycarbonyl)cytidine, capecitabine (1); A solution of NaOH (2 mg, 0.05 mmol) in water (0.5 mL) was slowly added to a solution of 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N4- (n-pentyloxycarbonyl)cytidine (20 mg, 0.045 mmol) in methanol (1.0 mL) at -15 oC. The reaction mixture was stirred at that temperature for 20 hours. It was taken to pH = 5 with HCl 5% keeping the temperature at -15°C and it was partitioned in CH2Cl2 (10 mL) and saturated solution of NaCl (5 mL). The organic phase was washed with a saturated solution of NaCl (5 mL), it was dried (Na2SO4), and the solvent was evaporated. 16 mg of capecitabine (98% yield) were obtained as a crystalline white solid. 1H RMN (CDCl3-DMSO-d6, 500 MHz) δ 0.91 (t, J = 6.9 Hz, 3H, H-5"), 1.36 (m, 7H, H-3", H-4", H-5'), 1.67 (quint, 2H, J = 6.6 Hz, H-2"), 3.69 (s a, 1H, H-3'), 4.03 (s a, 1H, H-4'), 4.09 (m, 1H, H-2'), 4.14 (s a, 2H, H-1 "), 4.80 (s a, 1H, 3'-OH), 5.44 (s a, 1H, 2'-OH), 5.70 (d, J = 2.3 Hz, H-1'), 7.85 (s a, 1H, H-6), 10.22 (s a, 0.61H, NH), 11.77 (s a, 0.39H, NH).
References:
EP2241556,2010,A1 Location in patent:Page/Page column 10
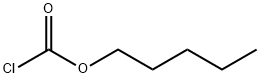
638-41-5
200 suppliers
$14.00/1g
161599-46-8
459 suppliers
$6.00/250mg

154361-50-9
819 suppliers
$5.00/1g
![Carbamic acid, N-[5-fluoro-1,2-dihydro-2-oxo-1-[(3aR,4R,6R,6aR)-tetrahydro-6-methyl-2-oxidofuro[3,4-d]-1,3,2-dioxathiol-4-yl]-4-pyrimidinyl]-, pentyl ester](/CAS/20210305/GIF/1309454-52-1.gif)
1309454-52-1
0 suppliers
inquiry

154361-50-9
819 suppliers
$5.00/1g

638-41-5
200 suppliers
$14.00/1g
66335-38-4
367 suppliers
$8.00/250mg

154361-50-9
819 suppliers
$5.00/1g
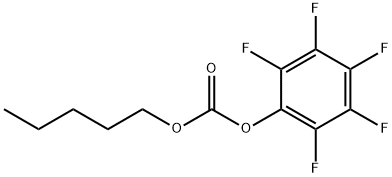
1332927-90-8
1 suppliers
inquiry

66335-38-4
367 suppliers
$8.00/250mg

154361-50-9
819 suppliers
$5.00/1g