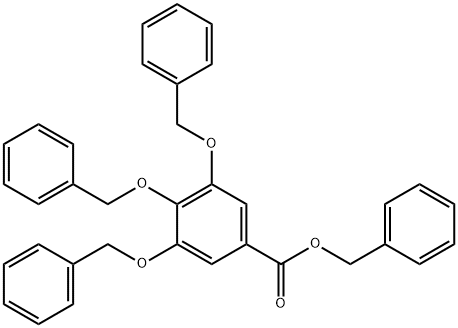
BENZYL TRI-BENZYLGALLOATE synthesis
- Product Name:BENZYL TRI-BENZYLGALLOATE
- CAS Number:475161-97-8
- Molecular formula:C35H30O5
- Molecular Weight:530.61
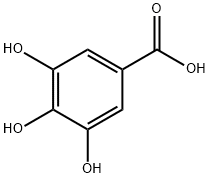
149-91-7
705 suppliers
$5.00/25g
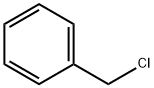
100-44-7
644 suppliers
$13.50/250G

475161-97-8
15 suppliers
$85.00/500mg
Yield:475161-97-8 86%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium carbonate in N,N-dimethyl-formamide at 100; for 4.5 h;
Steps:
Benzyl 3,4,5-Tribenzyloxybenzoate29) (8)
Benzyl 3,4,5-Tribenzyloxybenzoate29) (8) A mixture ofgallic acid monohydrate (1) (100 g, 0.532 mol), K2CO3 (587 g,4.25 mol), and nBu4NI (9.9 g, 26.8 mmol) in DMF (1 L) wasstirred using a mechanical stirrer at room temperature. BnCl(501 mL, 4.25 mol) was added to the suspention and the mixturewas heated at 100°C with stirring for 4.5 h. The reactionmixture was cooled to ambient temperature, poured into themixed solution of H2O (5 L) and hexane (0.5 L), and stirred.The precipitate was then collected on a glass filter and washedwith H2O and hexane. The obtained white solid (497.2 g) wasrecrystallized from acetone, providing 8 (243 g, 86%) as colorlessneedles; mp 101.3-102.8°C (acetone); 1H-NMR (500 MHz,CDCl3) δ: 5.10 (4H, s, CH2), 5.15 (2H, s, CH2), 5.33 (2H, s,CH2), 7.26-7.44 (22H, m, ArH); 13C-NMR (125 MHz, CDCl3)δ 66.8, 71.2, 75.1, 109.2, 125.2, 127.6, 128.0, 128.0, 128.1,128.2, 128.2, 128.6, 128.6, 136.1, 136.7, 137.5, 142.5, 152.6,165.9; IR (KBr) νmax 3064, 3032, 2945, 2866, 1710, 1594, 1500,1454, 1428, 1385, 1366, 1338, 1204, 1128, 1029, 971, 862, 753,730, 695, 604 cm-1; HR-MS (EI) Calcd for C35H30O5 [M]+:530.2093; Found: 530.2115 [M]+.
References:
Shioe, Kazuma;Ishikura, Shingo;Horino, Yoshikazu;Abe, Hitoshi [Chemical and Pharmaceutical Bulletin,2013,vol. 61,# 12,p. 1308 - 1314]

149-91-7
705 suppliers
$5.00/25g
100-39-0
434 suppliers
$10.00/10g

475161-97-8
15 suppliers
$85.00/500mg