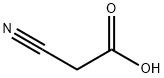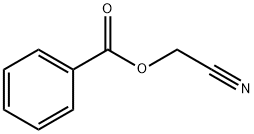
BENZOIC ACID CYANOMETHYL ESTER synthesis
- Product Name:BENZOIC ACID CYANOMETHYL ESTER
- CAS Number:939-56-0
- Molecular formula:C9H7NO2
- Molecular Weight:161.16
Yield:939-56-0 98.51%
Reaction Conditions:
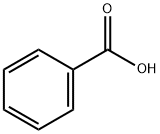
Stage #1: benzoic acidwith potassium carbonate in N,N-dimethyl-formamide;
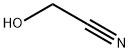
Stage #2: cyanomethyl bromide in N,N-dimethyl-formamide at 0 - 25;
Steps:
S3.1 [0175] Step 1: Synthesis of cyanomethyl benzoate (3a).
To a suspension of benzoic acid (500 g, 4.09 mol, 625.00 mL, 1 eq) in DMF (1500 mL), K2CO3 (700.00 g, 5.06 mol, 1.24 eq) was added and the mixture was stirred until the evolution of gas has ceased. The reaction mixture was cooled to 0°C and a solution of 2-bromoacetonitrile (491.10 g, 4.09 mol, 272.83 mL, 1 eq) in DMF (500 mL) was added drop-wise. The reaction mixture was stirred overnight (12 hr) at room temperature (25°C). TLC (Petroleum ether/Ethyl acetate=3/l) indicated the material was consumed completely and one new spot formed. The reaction mixture was filtered and diluted with H2O (3000 mL) and extracted with EA (2000 mL x 2). The combined organic layers were washed with brine (2000 mL x 2), dried over Na2SO4, filtered and concentrated under reduced pressure, the residue was purified by column chromatography (SiO2, Petroleum ether/Ethyl acetate=10/l to 5/1) to give cyanomethyl benzoate 3a (650 g, 4.03 mol, 98.51% yield) as an oil. 1H NMR (400 MHz, CHLOROFORM-d) δ = 8.11 - 8.03 (m, 2H), 7.67 - 7.60 (m, 1H), 7.53 - 7.45 (m, 2H), 4.97 (s, 2H).
References:
WO2022/56449,2022,A1 Location in patent:Paragraph 0174-0175
7127-19-7
119 suppliers
$17.00/5g

939-56-0
15 suppliers
inquiry