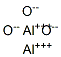
Aluminum oxide synthesis
- Product Name:Aluminum oxide
- CAS Number:1344-28-1
- Molecular formula:Al2O3
- Molecular Weight:101.96
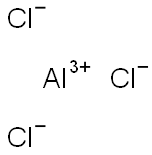
7446-70-0
717 suppliers
$15.00/25g
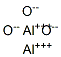
1344-28-1
542 suppliers
$15.00/25g
Yield:-
Reaction Conditions:
with air in solid;direct thermal decompn., evacuated at 600°C for 3 h; X-ray diffraction analysis;
References:
Utiyama, Masahiro;Hattori, Hideshi;Tanabe, Kozo [Bulletin of the Chemical Society of Japan,1981,vol. 54,# 8,p. 2521 - 2522]
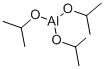
555-31-7
481 suppliers
$18.00/5g
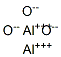
1344-28-1
542 suppliers
$15.00/25g
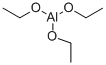
555-75-9
122 suppliers
$42.95/10g
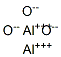
1344-28-1
542 suppliers
$15.00/25g
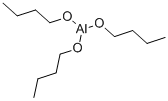
3085-30-1
67 suppliers
$57.00/25gm
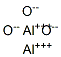
1344-28-1
542 suppliers
$15.00/25g
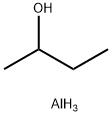
2269-22-9
218 suppliers
$10.00/5g
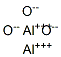
1344-28-1
542 suppliers
$15.00/25g