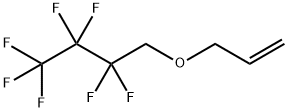
ALLYL 1H,1H-HEPTAFLUOROBUTYL ETHER synthesis
- Product Name:ALLYL 1H,1H-HEPTAFLUOROBUTYL ETHER
- CAS Number:648-42-0
- Molecular formula:C7H7F7O
- Molecular Weight:240.12
Yield:648-42-0 69%
Reaction Conditions:
with sodium hydroxide in neat (no solvent) at 80; for 7 h;Autoclave;
Steps:
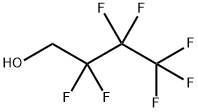
4.2.1 Synthesis of 4-(allyloxy)-1,1,1,2,2,3,3-heptafluorobutane (1)
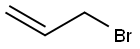
A flask was charged with perfluorinated alcohol C3F7CH2OH (40.0 g, 0.20 mol), allyl bromide (24.2 g, 0.20 mol) and grinded NaOH (8.00 g, 0.20 mol) and placed in the autoclave. The reaction was carried out for 7 h at 80 °C. The clean product 1 (33.6 g, 0.14 mol, Y = 69%) was obtained by fractional distillation as a colourless liquid (bp = 100-103 °C, d = 1.30 g/cm3). 1H NMR (300.1 MHz, CDCl3): δ 5.88 (ddt, 3Jtrans = 17.1 Hz, 3Jcis = 10.3 Hz, 3JH,H = 5.7 Hz, 1H, CH2CH=CH2), 5.30 (m, 2H, CH2CH=CH2), 4.14 (d, 3JH,H = 5.7 Hz, 2H, CH2CH=CH2), 3.91 (t, 3JH,F = 13.8 Hz, 2H, CF2CH2). 13C NMR (75.5 MHz, CDCl3): δ 133.0 (CH2CH=CH2), 118.7 (CH2CH=CH2), 120.5-105.5 (C3F7), 73.4 (CH2CH=CH2), 66.3 (t, 2JC,F = 25.8, CF2CH2). 19F NMR (282.3 MHz, CDCl3): δ -81.25 (t, 3JF,F = 9.5 Hz, CF3), -123.60 (m, CF2), -128.08 (s, CF2). MS (EI), m/z (rel. ab.%): 240 (M+°, 100%), 69 (CF3+°, 40%) 41 ([CH2-CH=CH2]+°, 70%).
References:
Lazzari, Dario;Cassani, Maria Cristina;Solinas, Gavino;Pretto, Marisa [Journal of Fluorine Chemistry,2013,vol. 156,p. 34 - 37]