ABBV-075 synthesis
- Product Name:ABBV-075
- CAS Number:1445993-26-9
- Molecular formula:C22H19F2N3O4S
- Molecular Weight:459.47
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1445993-26-9
93 suppliers
$25.00/500μg
Yield: 82%
Reaction Conditions:
with cetyltrimethylammonim bromide in tetrahydrofuran;water at 90; for 16 h;
Steps:
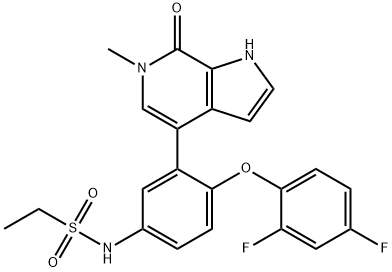
36; 36e N-[4-(2,4-difluorophenoxy)-3-(6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridin-4-yl)phenyl]ethanesulfonamide
A mixture of Example 36d (5.3 g, 7.5 mmol), potassium hydroxide (8.43 g, 150 mmol), and N,N,N-trimethylhexadecan-1-aminium bromide (0.137 g, 0.375 mmol) in tetrahydrofuran (60 mL) and water (30 mL) was heated at 90 °C for 16 hours. Tetrahydrofuran was removed under reduced pressure, and the residue was partitioned between water and ethyl acetate. The aqueous layer was neutalized to pH =7 using 10% HCl. The aqueous layer was then extracted with ethyl acetate. The combined organic layers were washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, filtered, and concentrated. The residue was purified by flash chromatography (silica gel, ethyl acetate). The desired fractions were combined and concentrated. The residue was triturated with 20 mL of acetonitrile to provide the title compound (2.82 g, 82 % yield). 1H NMR (300 MHz, DMSO-de) δ 1.23 (t, J = 7.3 Hz, 3H), 3.11 (q, J = 7.3 Hz, 2H), 3.53 (s, 3H), 6.27 - 6.22 (m, 1H), 6.91 (d, J = 8.7 Hz, 1H), 7.13 - 6.93 (m, 2H), 7.19 (dd, J = 8.8, 2.7 Hz, 1H), 7.32 - 7.25 (m, 2H), 7.42 - 7.31 (m, 2H), 9.77 (s, 1H), 12.04 (bs, 1H). MS (ESI+) m/z 460.1 (M+H)+.
References:
ABBVIE INC.;ABBOTT LABORATORIES TRADING (SHANGHAI) COMPANY, LTD.;WANG, Le;PRATT, John K.;MCDANIEL, Keith F.;DAI, Yujia;FIDANZE, Steven D.;HASVOLD, Lisa;HOLMS, James H.;KATI, Warren M.;LIU, Dachun;MANTEI, Robert A.;MCCLELLAN, William J;SHEPPARD, George S.;WADA, Carol K. WO2013/97601, 2013, A1 Location in patent:Page/Page column 110; 112
![4-(5-amino-2-(2,4-difluorophenoxy)phenyl)-6-methyl-1H-pyrrolo[2,3-c]pyridin-7(6H)-one](/CAS/20180601/GIF/1445994-28-4.gif)
1445994-28-4
1 suppliers
inquiry
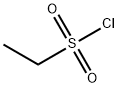
594-44-5
253 suppliers
$21.00/25g

1445993-26-9
93 suppliers
$25.00/500μg
![4-bromo-7-methoxy-1-tosyl-1H-pyrrolo[2,3-c]pyridine](/CAS/20180629/GIF/1445993-85-0.gif)
1445993-85-0
29 suppliers
inquiry

1445993-26-9
93 suppliers
$25.00/500μg
![4-bromo-1-tosyl-1H-pyrrolo[2,3-c]pyridin-7(6H)-one](/CAS/20180629/GIF/1445993-86-1.gif)
1445993-86-1
20 suppliers
inquiry

1445993-26-9
93 suppliers
$25.00/500μg
![4-bromo-6-methyl-1-tosyl-1H-pyrrolo[2,3-c]pyridin-7(6H)-one](/CAS/20200401/GIF/1445993-87-2.gif)
1445993-87-2
50 suppliers
$445.00/1g

1445993-26-9
93 suppliers
$25.00/500μg