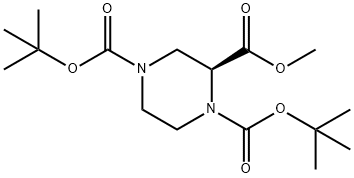
(S)-1,4-di-Boc-piperazine-2-carboxylic acid Methyl ester synthesis
- Product Name:(S)-1,4-di-Boc-piperazine-2-carboxylic acid Methyl ester
- CAS Number:958635-19-3
- Molecular formula:C16H28N2O6
- Molecular Weight:344.4

788799-69-9
59 suppliers
$42.00/100mg

74-88-4
353 suppliers
$15.00/10g

958635-19-3
34 suppliers
$59.00/250mg
Yield:958635-19-3 95%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 0 - 20;Inert atmosphere;
Steps:
1
To a suspension of solution of (S) - l ,4bis(tert-butoxycarbonyl)piperazine-2-carboxylic acid (18.3g, 45mmol) and potassium carbonate (8,2g; 59mmol) in DMF (150ml) cooled to 0 0C under N2, was added dropwise a solution of iodomethane (7.36ml; 1 18mmol). The reaction mixture was left to stir at room temperature for 18h. It was diluted with ethyl acetate, washed with water and brine. The organic was dried over magnesium sulfate, filtered and concentrated under reduced pressure to afford the title compound as a white solid (18g; 95%). 1H NMR (CDCl3) 1.46-1.50 (18H, d), 1.65 (3H, s), 2.80-2.90 (I H, m), 3.10-3.40 (2H, m), 3.75-4.10 (2H, m), 4.60- 4.70 (2H, m).
References:
WO2010/11772,2010,A2 Location in patent:Page/Page column 110; 111
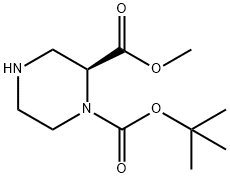
796096-64-5
128 suppliers
$31.00/100mg
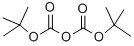
24424-99-5
864 suppliers
$13.50/25G

958635-19-3
34 suppliers
$59.00/250mg

24424-99-5
864 suppliers
$13.50/25G

958635-19-3
34 suppliers
$59.00/250mg
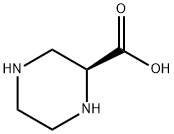
147650-70-2
161 suppliers
$15.00/250mg

958635-19-3
34 suppliers
$59.00/250mg