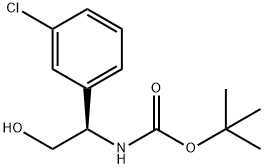
CarbaMic acid, N-[(1R)-1-(3-chlorophenyl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester synthesis
- Product Name:CarbaMic acid, N-[(1R)-1-(3-chlorophenyl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester
- CAS Number:926291-64-7
- Molecular formula:C13H18ClNO3
- Molecular Weight:271.74
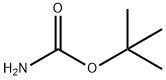
4248-19-5
380 suppliers
$5.00/5g
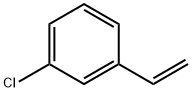
2039-85-2
174 suppliers
$11.00/1g
![CarbaMic acid, N-[(1R)-1-(3-chlorophenyl)-2-hydroxyethyl]-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/926291-64-7.gif)
926291-64-7
22 suppliers
$19.00/100mg
Yield:926291-64-7 51%
Reaction Conditions:
Stage #1: tert-butyl carbazatewith sodium hydroxide;tert-butyl hypochloride in propan-1-ol at 15; for 0.166667 h;
Stage #2: 3-Chlorostyrenewith (DHQD)2PHAL;potassium osmate(VI) in propan-1-ol at 0;
Steps:
J
To a stirred solution of t-butyl carbamate (2.0 equivalents) in n-propanol (4 L/mol) in a cold-water bath (~ 15°C) was added IN NaOH (2.05 equivalents), and t-butyl hypochlorite (2.3 equivalents). The mixture was stirred for 10 min and then cooled to 00C. Solutions of (DHQD)2PHAL (0.02 equivalents) in n-propanol (4 L/mol), styrene (1 equivalent) in n- propanol (8 L/mol), and K2OsO4-2H2O (0.015 equivalents) were added sequentially. The mixture was stirred at 00C until the reaction was complete. The reaction was quenched by the addition of an aqueous Na2SO3 solution until any remaining green color was reduced to yellow/brown. The mixture was concentrated under reduced pressure. In a standard workup, the reaction mixture was partitioned between water and CH2Cl2. The phases were separated and the aqueous extracted with CH2Cl2. The combined organic extracts were dried (Na2SO4 or MgSO4), filtered and concentrated under reduced pressure. The crude material was purified by flash column chromatography on silica gel.; Using general procedure J, 3-chlorostyrene (28.0 g, 200 mmol) was converted to [(R)-l-(3-chloro-phenyl)-2-hydroxy-ethyl]-carbamic acid tert-butyl ester (27.9g, 51% yield, 99% purity by HPLC, 93.7%ee by HPLC). 1H NMR (CD3OD) δ 3.63-3.72 (m, 2H), 4.63-4.67 (m, IH), 7.26-7.37 (m, 2H), 7.43 (s, IH).
References:
WO2007/22371,2007,A2 Location in patent:Page/Page column 27; 31