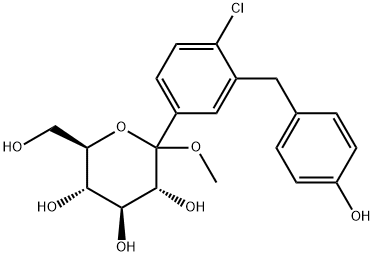
D-Glucopyranoside, methyl 1-C-[4-chloro-3-[(4-hydroxyphenyl)methyl]phenyl]- synthesis
- Product Name:D-Glucopyranoside, methyl 1-C-[4-chloro-3-[(4-hydroxyphenyl)methyl]phenyl]-
- CAS Number:912917-83-0
- Molecular formula:C20H23ClO7
- Molecular Weight:410.85
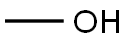
67-56-1
751 suppliers
$9.00/25ml
![Silane,[4-[(5-broMo-2-chlorophenyl)Methyl]phenoxy](1,1-diMethylethyl)diMethyl-](/CAS/20150408/GIF/864070-19-9.gif)
864070-19-9
31 suppliers
inquiry
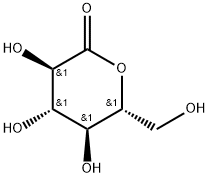
90-80-2
554 suppliers
$5.00/25g
![D-Glucopyranoside, methyl 1-C-[4-chloro-3-[(4-hydroxyphenyl)methyl]phenyl]-](/CAS/20210111/GIF/912917-83-0.gif)
912917-83-0
2 suppliers
inquiry
Yield:912917-83-0 84.8%
Reaction Conditions:
Stage #1: [4-(5-bromo-2-chloro-benzyl)-phenoxy]-tert-butyl-dimethyl-silanewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Stage #2: D-Glucono-1,5-lactone in tetrahydrofuran;hexane;toluene at -78; for 1.5 h;Inert atmosphere;
Stage #3: methanolwith methanesulfonic acid in tetrahydrofuran;hexane;toluene at 20; for 18 h;Inert atmosphere;
Steps:
7 Synthesis of (3R,4S,5S,6R)-2-(4-chloro-3-(4-hydroxybenzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol (7)
56.36 g (0.13 mol) at -78 °C 6 is added to 300 mL of tetrahydrofuran under nitrogen protection In a solution, 2.3 mL (0.18 mol) of n-butyl hexane solution was slowly added dropwise to the mixture. After stirring the reaction for 30 min, the reaction droplets were added to the pre-cooling under the protection of nitrogen. -78 ° C 3 (80.10g, 0.18mol) in toluene solution, stirring reaction for 1.5h, then adding methanesulfonic acid in methanol solution (250mL, 0.6mol / L), Slowly rise to room temperature and react for 18 h. Quench the reaction by adding 65 mL of saturated sodium bicarbonate solution. The organic layer was combined and dried over anhydrous sodium sulfate. 7 crude product was obtained, dissolved in hot toluene solution, slowly added to hexane solution, and a yellow solid was precipitated, 77.8 g, yield 84.8%.
References:
CN108285439,2018,A Location in patent:Paragraph 0038; 0109-0111
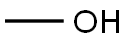
67-56-1
751 suppliers
$9.00/25ml
![D-Glucopyranoside, methyl 1-C-[4-chloro-3-[(4-hydroxyphenyl)methyl]phenyl]-](/CAS/20210111/GIF/912917-83-0.gif)
912917-83-0
2 suppliers
inquiry
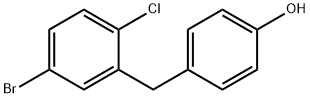
864070-18-8
176 suppliers
$35.00/1g
![D-Glucopyranoside, methyl 1-C-[4-chloro-3-[(4-hydroxyphenyl)methyl]phenyl]-](/CAS/20210111/GIF/912917-83-0.gif)
912917-83-0
2 suppliers
inquiry
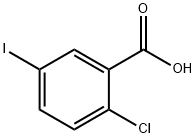
19094-56-5
525 suppliers
$6.00/5g
![D-Glucopyranoside, methyl 1-C-[4-chloro-3-[(4-hydroxyphenyl)methyl]phenyl]-](/CAS/20210111/GIF/912917-83-0.gif)
912917-83-0
2 suppliers
inquiry
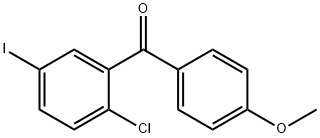
1459754-39-2
14 suppliers
inquiry
![D-Glucopyranoside, methyl 1-C-[4-chloro-3-[(4-hydroxyphenyl)methyl]phenyl]-](/CAS/20210111/GIF/912917-83-0.gif)
912917-83-0
2 suppliers
inquiry