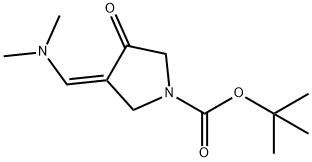
(Z)-tert-butyl 3-((dimethylamino)methylene)-4-oxopyrrolidine-1-carboxylate synthesis
- Product Name:(Z)-tert-butyl 3-((dimethylamino)methylene)-4-oxopyrrolidine-1-carboxylate
- CAS Number:905274-02-4
- Molecular formula:C12H20N2O3
- Molecular Weight:240.3

4637-24-5
612 suppliers
$5.00/25g
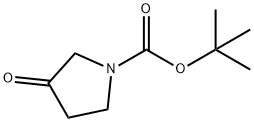
101385-93-7
479 suppliers
$5.00/1g

905274-02-4
14 suppliers
inquiry
Yield:905274-02-4 82%
Reaction Conditions:
in tetrahydrofuran at 70; for 16 h;
Steps:
1 Synthesis 1 : tert- Butyl (Z)-3-((dimethylamino)methylene)-4- oxopyrrolidine-1 -carboxylate (ZCN004)
to a solution of A/-Boc-3-pyrrolidinone (1 1 g, 59.4 mmol), N,N- dimethylformamide dimethyl acetal (21.2 g, 178.2 mmol, 23.7 mL) in THF (20 mL) was stirred at 70 °C for 16 h. The reaction was cooled down to 23 °C and the solvent was evaporated under reduced pressure. The residue was triturated with hexane, filtered, washed with hexane and dried under reduced pressure. The resulting enamine ZCN004 as a yellow-orange powder (1 1.7 g, 82%), which was used for the next synthetic step without further purification. 1H NMR (400 MHz, CDC ) d 7.32 (s, (0283) 1 H), 4.59 (s, 2H), 3.82 (s, 2H), 3.1 1 (s, 6H), 1.49 (s, 9H). m/z (ESI) (relative intensity) 241.1 [M+H]+ (100).
References:
WO2020/178782,2020,A1 Location in patent:Paragraph 0169

101385-93-7
479 suppliers
$5.00/1g

905274-02-4
14 suppliers
inquiry