
2-METHYL-3-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)BENZOIC ACID synthesis
- Product Name:2-METHYL-3-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)BENZOIC ACID
- CAS Number:882678-82-2
- Molecular formula:C14H19BO4
- Molecular Weight:262.11
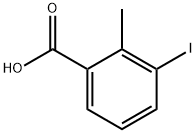
133232-56-1
137 suppliers
$12.00/1g
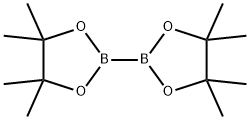
73183-34-3
582 suppliers
$6.00/5g

882678-82-2
18 suppliers
inquiry
Yield:882678-82-2 55%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in dimethyl sulfoxide at 80;Inert atmosphere;
Steps:
10.1 2-Methyl-3 -(4,4,5,5 -tetramethyl-[ 1,3 ,2]dioxaborolan-2-yl)-benzoic acid.
To a 300 ml round bottom three neck flask equipped with condenser, magnetic stirrer and an inert-gas outlet, were added 3-iodo-2-Methyl-Benzoic acid (10 g, 38.2mniol), BisQ,inacolato)diboron (11.64 g, 46.Ommol), potassium acetate (11.23 g,112.Ommol) and 100 In of anhydrous DMSO. The flask was purged with nitrogen for15-20 mm. The catalyst dichioro [1, 1 ‘-bis(diphenylphosphino)ferrocene]palladium dichloroinethane (0.85 g, 1.lmmol) was added through an open neck under slight positive nitrogen flow. The reaction mixture was stirred at 80°C overnight. The reaction mixture was allowed to cool then poured into 500 ml of water. After 1 hour of stirring at room temperature, a brownish precipitant was filtered onto fritted filter funnel and washed with water. The precipitant was redissolved in 200 ml of ether and filtered through a 1” thick layer of celite to remove traces of Pd. The filter cake was washed with 100 ml ether. The combined etherial solution was washed with 4 x 200 ml of 2N NaOH. The water phases were combined and acidified with 6N HC1 until pH - 5-6 (approx 100 ml). A white precipitant was liltered onto fitted filter, washed with200 ml of water, and dried in a vacuum oven at 60°C for 2 hours. The filtrate was placed into fridge for overnight, and a second crop of white precipitant was isolated via filtration. Overall amount of product obtained was 5.5 g (55% yield). ‘H NMR (CDC13, 300 MHz) 8.05 (d, 1H), 7.9 (d, 1H), 7.25 (t, IH), 2.6 (s, 3H), 1.4 (s, 12H).
References:
WO2014/144380,2014,A1 Location in patent:Paragraph 0530
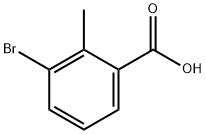
76006-33-2
319 suppliers
$10.00/5g

73183-34-3
582 suppliers
$6.00/5g

882678-82-2
18 suppliers
inquiry