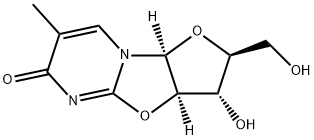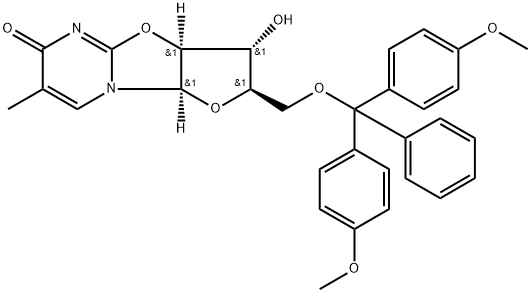
5'-O-(4,4'-Dimethoxytrityl)-5-methyl-2,2'-anhydro-D-uridine synthesis
- Product Name:5'-O-(4,4'-Dimethoxytrityl)-5-methyl-2,2'-anhydro-D-uridine
- CAS Number:817623-11-3
- Molecular formula:C31H30N2O7
- Molecular Weight:542.59
Yield:817623-11-3 97.8%
Reaction Conditions:
with pyridine;dmap at 0 - 5; for 1.5 h;
Steps:
24
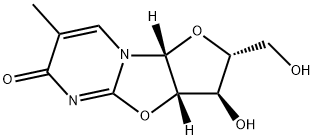
To a previously cooled (0-5° C.) mixture of 2,2'-anhydro-1--D-arabinofuranosyl)-thymine 1 (2.40 g, 10.0 mmol) and DMAP (111 mg, 0.9 mmol) in anhydrous pyridine (15 mL), was added 4,4'-dimethoxytrityl chloride (3.56 g, 10.5 mmol) portionwise over a period of 3 minutes. The resulting mixture was kept stirring at 0-5° C. under argon and after 1.5 h, t.l.c. analysis (silica plate, 1:9 methanol:dichloromethane) indicated no remaining starting material. The reaction mixture was concentrated in vacuo at 45° C. The residue was taken into dichloromethane (50 mL) and sat. sodium bicarbonate solution (20 mL). After stirring at room temperature for 10 minutes, the layers were separated and the organic layer was washed with distilled water (2×20 mL) and dried with anhydrous sodium sulfate. The reaction mixture was concentrated in vacuo at 50° C. and residue was co-evaporated with toluene (2×10 mL). The resulting crude residue was triturated with dichloromethane (5 mL) and TBME (25 mL). After stirring at room temperature for 1 hour, the solid was collected by filtration under reduced pressure and washed with TBME (15 mL). The yellow solid was dried under vacuum affording 2,2'-anhydro-1-(5-O-dimethoxytrityl--D-arabinofuranosyl)thymine 2 (5.3 g, 97.8% yield, 91% AUC (area under curve) by HPLC analysis).
References:
US2005/59632,2005,A1 Location in patent:Page/Page column 41; 42
22423-26-3
202 suppliers
$9.00/1g
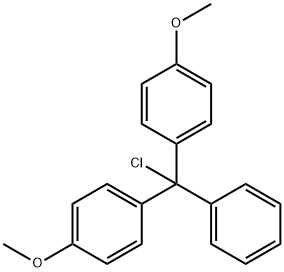
40615-36-9
562 suppliers
$5.00/1g

817623-11-3
12 suppliers
inquiry
1463-10-1
410 suppliers
$5.00/250mg

817623-11-3
12 suppliers
inquiry
3180-76-5
1 suppliers
inquiry

817623-11-3
12 suppliers
inquiry
65-71-4
533 suppliers
$5.00/5g

817623-11-3
12 suppliers
inquiry