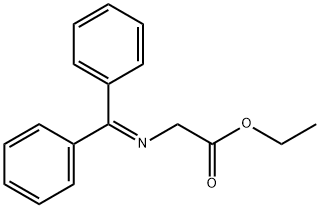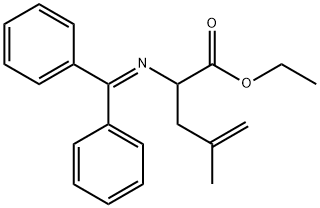
2-[(Diphenylmethylene)amino]-4-methyl-4-pentenoic acid ethyl ester synthesis
- Product Name:2-[(Diphenylmethylene)amino]-4-methyl-4-pentenoic acid ethyl ester
- CAS Number:80741-44-2
- Molecular formula:C21H23NO2
- Molecular Weight:321.41
Yield:80741-44-2 99%
Reaction Conditions:
with lithium hexamethyldisilazane in tetrahydrofuran;ethyl acetate;
Steps:
1.A A.
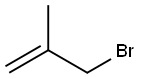
A. Ethyl (2RS)-2-Diphenylmethylenamino-4-methyl-4-pentenoate To a stirred solution of N-(diphenylmethylene)glycine ethyl ester (available from Aldrich Chemical Co., Milwaukee, Wis.) (50.0 g, 187 mmol) in dry THF (200 mL) at -78° C. under argon was added a 1.0 M solution of lithium bis(trimethylsilyl)amide (Li HMDS; 210 mL, 0.2 mol) in THF over a period of 45 min ("1.A, Scheme A). After 0.5 hour at -78° C., 3-bromo-2-methylpropene (18.91 mL, 187 mmol) was added dropwise to the orange-yellow suspension. After the addition was complete, the reaction mixture was stirred for an additional 0.5 hour at 0° C. and then allowed to warm to room temperature. After 1 hour the reaction mixture was concentrated under reduced pressure to give an orange-colored oil. The crude material was dissolved in ethyl acetate (EA) (600 mL), washed with brine (100 mL), dried over anhydrous MgSO4 and concentrated under reduced pressure to give the desired compound (60 g, 99%), which was used for the next step without further purification. 1H NMR (CDCl3, 300 MHz), δ 7.1-7.7 (m, 10H), 4.72 (dd, 2H), 4.2 (m, 3H), 2.63 (m, 2H), 1.5 (s, 3H), 1.27 (t, 3H).
References:
US6617426,2003,B1