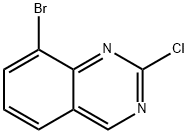
8-BROMO-2-CHLOROQUINAZOLINE synthesis
- Product Name:8-BROMO-2-CHLOROQUINAZOLINE
- CAS Number:956100-63-3
- Molecular formula:C8H4BrClN2
- Molecular Weight:243.49
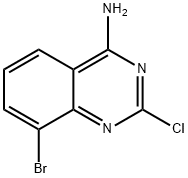
956100-62-2
82 suppliers
$27.00/1g

956100-63-3
124 suppliers
$55.00/250mg
Yield: 67%
Reaction Conditions:
with isopentyl nitrite in tetrahydrofuran at 60; for 8.67 h;
Steps:
L; 195d
To a stirred mixture of 8-bromo-2-chloroquinazolin-4-amine (12.93 g, 50 mmol) in THF (500 mL) was added isoamyl nitrite (23.43 g, 200 mmol) over 3 hours and 40 mins at 6O0C. The mixture was stirred for another 5 hours. TLC (PE:EA,1 :1) showed reaction complete. After cooling to room temperature, the
References:
PFIZER PRODUCTS INC. WO2007/125405, 2007, A2 Location in patent:Page/Page column 25; 81-82
331647-05-3
118 suppliers
$18.00/100mg

956100-63-3
124 suppliers
$55.00/250mg
20776-51-6
261 suppliers
$6.00/1g

956100-63-3
124 suppliers
$55.00/250mg
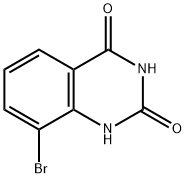
331646-99-2
77 suppliers
$11.00/250mg

956100-63-3
124 suppliers
$55.00/250mg

331646-99-2
77 suppliers
$11.00/250mg

956100-63-3
124 suppliers
$55.00/250mg