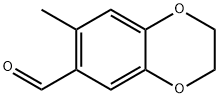
7-methyl-2,3-dihydro-1,4-benzodioxine-6-carbaldehyde(SALTDATA: FREE) synthesis
- Product Name:7-methyl-2,3-dihydro-1,4-benzodioxine-6-carbaldehyde(SALTDATA: FREE)
- CAS Number:724791-20-2
- Molecular formula:C10H10O3
- Molecular Weight:178.18
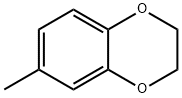
33632-35-8
6 suppliers
inquiry
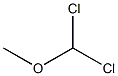
4885-02-3
188 suppliers
$10.00/1g

724791-20-2
21 suppliers
$60.00/100mg
Yield:724791-20-2 79%
Reaction Conditions:
Stage #1: 6-methyl-2,3-dihydrobenzo[b][1,4]dioxine;Dichloromethyl methyl ether in 1,2-dichloro-ethane; for 1 h;
Stage #2: with tin(IV) chloride at 20;
Stage #3: with hydrogenchloride;water in 1,2-dichloro-ethane;
Steps:
9.B
Step B: Synthesis of 7-methyl-2,3-dihydrobenzo[b][1,4]dioxine-6-carbaldehyde; A mixture of 6-methyl-2,3-dihydrobenzo[b][1,4]dioxine (6.4 g, 42.8 mmol) and 1,2-dichloroethane (250 mL) was cooled to 0° C. and then stirred for 1 h. After this time, dichloromethyl methyl ether (11.5 mL, 128.5 mmol) was then added and the reaction stirred for 1 h then, tin (IV) chloride (7.52 mL, 64.3 mmol) was added portion wise to the reaction over 3 h, and the reaction then allowed to warm to room temperature overnight. After this time, the reaction was poured into 3 N hydrochloric acid (150 mL) and the organic layer extracted three times with dichloromethane (100 mL). The combined organic extracts were then dried over magnesium sulfate, filtered, and concentrated under reduced pressure to afford 7-methyl-2,3-dihydrobenzo[b][1,4]dioxine-6-carbaldehyde (4.8 g, 79%) as an off-white solid; 1H NMR(500 MHz, CDCl3) δ 10.07 (s, 1H), 7.33 (s, 1H), 6.72 (s, 1H), 4.28 (m, 4H), 2.55 (s, 3H).
References:
US2009/312323,2009,A1 Location in patent:Page/Page column 18