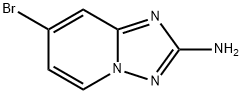
7-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-amine synthesis
- Product Name:7-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-amine
- CAS Number:882521-63-3
- Molecular formula:C6H5BrN4
- Molecular Weight:213.03
![[[(4-Bromo-2-pyridinyl)amino]thioxomethyl]-carbamic acid ethyl ester](/CAS/20180703/GIF/882521-62-2.gif)
882521-62-2
3 suppliers
inquiry
![7-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-amine](/CAS/GIF/882521-63-3.gif)
882521-63-3
111 suppliers
$30.00/250mg
Yield: 87.4%
Reaction Conditions:
with hydroxylamine hydrochloride;N-ethyl-N,N-diisopropylamine in ethanol at 25; for 24 h;Product distribution / selectivity;Reflux;
Steps:
35.b
A mixture of hydroxylamine hydrochloride (20.0 g, 288 mmol) and N-ethyldiisopropylamine (30.1 ml, 173 mmol) in ethanol (367 ml) is stirred for a few minutes at room temperature and the mixture is added to l-ethoxycarbonyl-3-(4-bromo-pyridin-2-yl)-thiourea (17.5 g, 57.5 mmol). The resulting mixture is refluxed for 1 day. The solvent is evaporated and 100 ml water is added to the residue. The suspension is stirred for 10 minutes, the solid is collected by filtration, washed with water and dried affording 7-bromo-[l,2,4]triazolo[l,5-a]pyridin-2-amine (10.71 g, 87.4%) as a light yellow solid. Mp.: 190-2°C. MS: m/z= 213.0, 215.0 (M+H+).
References:
F. HOFFMANN-LA ROCHE AG;FLOHR, Alexander;GOBBI, Luca;GROEBKE ZBINDEN, Katrin;KOERNER, Matthias;PETERS, Jens-Uwe WO2012/76430, 2012, A1 Location in patent:Page/Page column 90
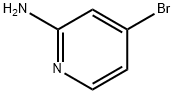
84249-14-9
370 suppliers
$6.00/1g
![7-bromo-[1,2,4]triazolo[1,5-a]pyridin-2-amine](/CAS/GIF/882521-63-3.gif)
882521-63-3
111 suppliers
$30.00/250mg