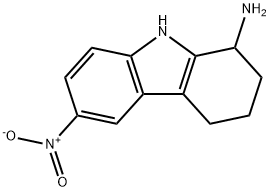
6-NITRO-2,3,4,9-TETRAHYDRO-1H-CARBAZOL-1-AMINE synthesis
- Product Name:6-NITRO-2,3,4,9-TETRAHYDRO-1H-CARBAZOL-1-AMINE
- CAS Number:337352-79-1
- Molecular formula:C12H13N3O2
- Molecular Weight:231.25
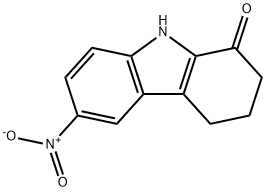
124253-88-9
2 suppliers
inquiry

337352-79-1
6 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 6-nitro-1,2,3,4-tetrahydro-9H-carbazol-1-onewith ammonium acetate in methanol at 23;
Stage #2: with sodium cyanoborohydride in methanol at 60;
Steps:
General procedure to prepare racemic amines iv (1-20)
General procedure: Solid NH4OAc (5.0 mmol) was added to a stirring solution of requisite ketone iii (0.5 mmol) dissolved in 20 mL solvent grade CH3OH, and this mixture was allowed to stir at room temperature (23 °C) for 3-6 h. Upon complete consumption of iii, as determinedby thin layer chromatography, solid NaCNBH3 (2.5 mmol) was added andthe temperature was raised to 60 °C. After stirring 12-16 h, the reaction was cooled to room temperature (23 °C) and treated with an aqueous 1 M HCl solution. The mixture was extracted with ethyl acetate, and the combined organic layers were washed with brine and dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to afford the crude product. The desired product was purified by flash column chromatography (SiO2) using CH2Cl2 and CH3OH as the eluent. NMR of products were compared to literature values to confirm structure.
References:
Li, Yangxiong;Gardner, Jessi J.;Fortney, Katherine R.;Leus, Inga V.;Bonifay, Vincent;Zgurskaya, Helen I.;Pletnev, Alexandre A.;Zhang, Sheng;Zhang, Zhong-Yin;Gribble, Gordon W.;Spinola, Stanley M.;Duerfeldt, Adam S. [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 14,p. 1836 - 1841] Location in patent:supporting information