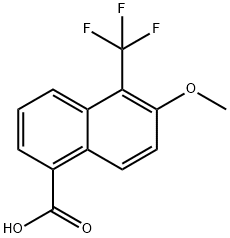
6-METHOXY-5-(TRIFLUOROMETHYL)-1-NAPHTHOIC ACID synthesis
- Product Name:6-METHOXY-5-(TRIFLUOROMETHYL)-1-NAPHTHOIC ACID
- CAS Number:84532-72-9
- Molecular formula:C13H9F3O3
- Molecular Weight:270.2
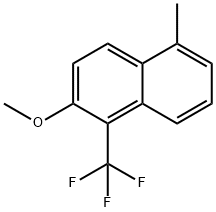
85674-78-8
0 suppliers
inquiry

84532-72-9
18 suppliers
inquiry
Yield:84532-72-9 55%
Reaction Conditions:
with sodium hydroxide;potassium permanganate in water;tert-butyl alcohol;
Steps:
8 Procedure A
EXAMPLE 8 6-Methoxy-5-(trifluoromethyl)-1-naphthalenecarboxylic acid (VIII, R=CH3) Procedure A A solution of KMnO4 (1.58 g, 10 mmol) in water (15 mL) was added dropwise over a period of 6 hr to a refluxing solution of 2-methoxy-5-methyl-1-(trifluoromethyl)naphthalene (500 mg, 2.1 mmol) in water/tert-butanol (11.8:88.2; 10 mL). The mixture was heated at reflux for an additional one hour and, while still hot, the mixture was filtered. The solid, collected on the filter, was washed with hot water and ethyl acetate. The combined filtrate and washings were concentrated under reduced pressure to a volume of about 3 mL. Water (20 mL) and 0.5 N aqueous NaOH (10 mL) were added to the residue. The mixture was extracted with diethyl ether. After drying (MgSO4) and evaporating of the extract, 140 mg of starting material was obtained. The aqueous layer from the preceding extraction was rendered acidic with 1 N aqueous H2 SO4. The resulting precipitate was collected, washed on the filter with water and dried over P2 O5 under reduced pressure for 18 hr to give the title compound (222 mg, 55% yield based on recovered starting material). The product had mp 221°-222° C.
References:
US4408077,1983,A
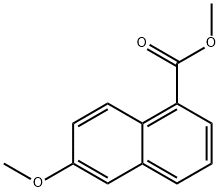
61109-48-6
3 suppliers
inquiry

84532-72-9
18 suppliers
inquiry
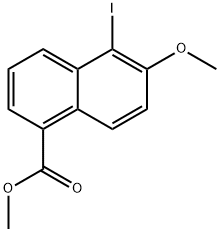
84532-68-3
0 suppliers
inquiry

84532-72-9
18 suppliers
inquiry
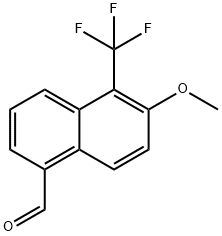
88245-15-2
0 suppliers
inquiry

84532-72-9
18 suppliers
inquiry
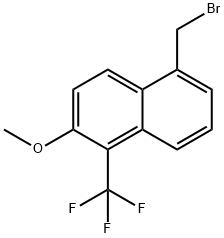
88245-14-1
0 suppliers
inquiry

84532-72-9
18 suppliers
inquiry