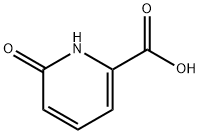
6-Hydroxypicolinic acid synthesis
- Product Name:6-Hydroxypicolinic acid
- CAS Number:19621-92-2
- Molecular formula:C6H5NO3
- Molecular Weight:139.11
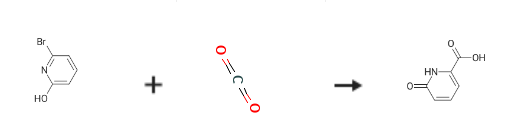
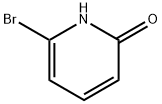
To a solution of 2-bromo-6-hydroxypyridine (0.76 g, 4.4 mmol,1.0 equiv.) in dry THF (20 mL) at 0 C was added a 2 M solution of -PrMgCl in THF (2.2 mL,4.4 mmol, 1.0 equiv.) during 5 min. The clear solution was stirred at that temperature for anadditional 5 min, and a 2.5 M solution of n-BuLi in hexanes (3.5 mL, 8.8 mmol, 2.0 equiv.) wasadded dropwise during 5min, while maintaining the temperature below 20 C. The resultingmixture was stirred at that temperature for 0.5 h, dry CO2 (0.20 g, 1.0 equiv.) was added to20 C. The resulting mixture was warmed to 20 C in 0.5 h and quenched with water (6 mL).After stirring the mixture below 20 C for 10 min, the phases were separated and the water phasewas extracted one additional time with ethyl acetate. The resulting suspension was allowed to reachroom temperature and fitered through a mbox0.5 1 cm pad of silica gel eluted with 10 mL of ethylacetate. The ?ltrate was concentrated and the residue was puri?ed by ash chromatography on silicagel (eluent: petroleum ether/ethyl acetate = 10:1) to afford product 3m as off-white solid, 0.56 g (yield: 93percent) , m.p.: 275–277 C. 1H-NMR (600 MHz, DMSO) 7.56 (dd, J = 8.9, 7.0 Hz, 1H), 6.97 (d, J = 6.8 Hz,1H), 6.65 (d, J = 9.0 Hz, 1H). 13C-NMR (151 MHz, DMSO) 163.28, 162.67, 140.51, 137.97, 123.88, 110.42.
27992-32-1
168 suppliers
$9.00/250mg
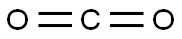
124-38-9
130 suppliers
$175.00/23402

19621-92-2
243 suppliers
$7.00/1g
Yield:19621-92-2 93%
Reaction Conditions:
Stage #1:6-bromo-2-hydroxypyridine with isopropylmagnesium chloride in tetrahydrofuran at 0; for 0.166667 h;
Stage #2: with n-butyllithium in tetrahydrofuran;hexane at -20; for 0.5 h;
Stage #3:carbon dioxide in tetrahydrofuran;hexane at -20; for 0.5 h;
Steps:
6-Hydroxy-pyridine-2-carboxylic acid (3m)
To a solution of 2-bromo-6-hydroxypyridine (0.76 g, 4.4 mmol,1.0 equiv.) in dry THF (20 mL) at 0 C was added a 2 M solution of -PrMgCl in THF (2.2 mL,4.4 mmol, 1.0 equiv.) during 5 min. The clear solution was stirred at that temperature for anadditional 5 min, and a 2.5 M solution of n-BuLi in hexanes (3.5 mL, 8.8 mmol, 2.0 equiv.) wasadded dropwise during 5min, while maintaining the temperature below 20 C. The resultingmixture was stirred at that temperature for 0.5 h, dry CO2 (0.20 g, 1.0 equiv.) was added to20 C. The resulting mixture was warmed to 20 C in 0.5 h and quenched with water (6 mL).After stirring the mixture below 20 C for 10 min, the phases were separated and the water phasewas extracted one additional time with ethyl acetate. The resulting suspension was allowed to reachroom temperature and fitered through a mbox0.5 1 cm pad of silica gel eluted with 10 mL of ethylacetate. The ltrate was concentrated and the residue was puried by ash chromatography on silicagel (eluent: petroleum ether/ethyl acetate = 10:1) to afford product 3m as off-white solid, 0.56 g (yield: 93%) , m.p.: 275-277 C. 1H-NMR (600 MHz, DMSO) 7.56 (dd, J = 8.9, 7.0 Hz, 1H), 6.97 (d, J = 6.8 Hz,1H), 6.65 (d, J = 9.0 Hz, 1H). 13C-NMR (151 MHz, DMSO) 163.28, 162.67, 140.51, 137.97, 123.88, 110.42.
References:
Tian, Qingqiang;Shang, Suqin;Wang, Huajun;Shi, Guoqiang;Li, Zhiyao;Yuan, Jianyong [Molecules,2017,vol. 22,# 11,art. no. 1952] Location in patent:supporting information
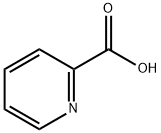
98-98-6
640 suppliers
$5.00/10g

19621-92-2
243 suppliers
$7.00/1g
672-67-3
16 suppliers
$310.00/250mg

19621-92-2
243 suppliers
$7.00/1g
499-83-2
643 suppliers
$6.00/10g

19621-92-2
243 suppliers
$7.00/1g