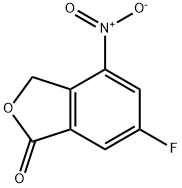
6-Fluoro-4-nitro-3H-isobenzofuran-1-one synthesis
- Product Name:6-Fluoro-4-nitro-3H-isobenzofuran-1-one
- CAS Number:1207453-90-4
- Molecular formula:C8H4FNO4
- Molecular Weight:197.12
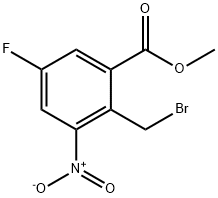
850462-65-6
22 suppliers
inquiry

1207453-90-4
95 suppliers
$30.00/1g
Yield:1207453-90-4 79%
Reaction Conditions:
with water in 1,4-dioxane; for 96 h;Reflux;
Steps:
63B
A mixture of methyl 5-fluoro-2-methyl-3-nitrobenzoate (28 g, 130.5 mmol), NBS (27.8 g, 156.6 mmol) and BPO (3.13 g, 13.1 mmol) in CCl4 (400 mL) was heated to reflux overnight. TLC (petroleum ether/EtOAc=15:1) showed the starting material was consumed completely. Water (200 mL) was added and CCl4 was removed under reduced pressure. The residue was extracted with DCM (200 mL×3). The combined organic layers were washed with brine, dried over Na2SO4, concentrated to give crude methyl 2-(bromomethyl)-5-fluoro-2-methyl-3-nitrobenzoate (36 g, yield 94%) as a brown oil. A mixture of methyl 2-(bromomethyl)-5-fluoro-2-methyl-3-nitrobenzoate (36 g, 123 mmol) in 1,4-dioxane (250 mL) and water (62.5 mL) was heated to reflux for 4 days. TLC (petroleum ether/EtOAc=15:1) showed the starting material was consumed completely. Dioxane was removed under reduced pressure. The residue was extracted with EtOAc (300 mL×4). The combined organic layers were washed with brine, dried over Na2SO4, concentrated to give crude product. The crude product was purified by gel chromatography (petroleum ether to petroleum ether/EtOAc=5:1) to give 6-fluoro-4-nitroisobenzofuran-1(3H)-one (19.2 g, yield 79%) as a white solid. 1H-NMR (400 MHz, CDCl3) δ (ppm): 5.74 (s, 2H), 7.97-7.98 (dd, 1H), 8.24-8.27 (dd, 1H); LC-MS (ESI) m/z: 198(M+1)+
References:
US2010/35883,2010,A1 Location in patent:Page/Page column 1
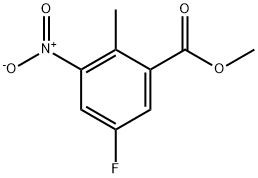
697739-03-0
166 suppliers
$8.00/250mg

1207453-90-4
95 suppliers
$30.00/1g
33184-16-6
321 suppliers
$6.00/1g

1207453-90-4
95 suppliers
$30.00/1g
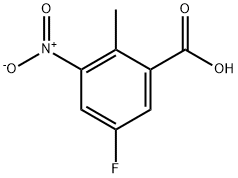
850462-64-5
96 suppliers
$11.00/250mg

1207453-90-4
95 suppliers
$30.00/1g