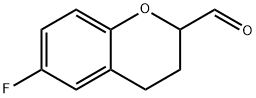
6-Fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran synthesis
- Product Name:6-Fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran
- CAS Number:99199-90-3
- Molecular formula:C11H11FO2
- Molecular Weight:194.2
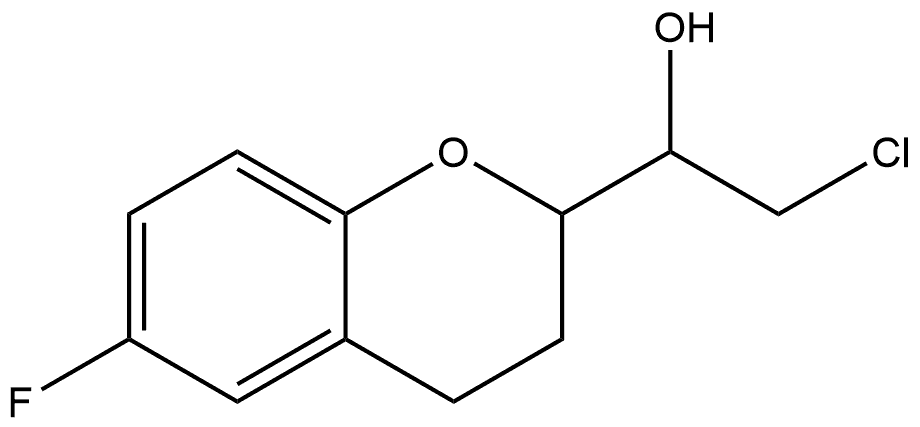
1017878-67-9
3 suppliers
inquiry

99199-90-3
109 suppliers
inquiry
Yield:99199-90-3 96%
Reaction Conditions:
Stage #1: 2-chloro-1-(6-fluoro-3,4-dihydro-2H-1-benzopyran-2yl)ethanolwith sodium hydroxide in water;isopropyl alcohol at 0; for 1.5 h;Inert atmosphere;
Stage #2: with acetic acid in water;isopropyl alcohol;toluene;
Steps:
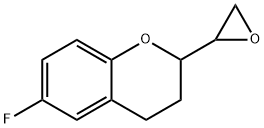
5 Synthesis of 6-fluoro-2-oxiranyl-1-benzopyran
Example 5 Synthesis of 6-fluoro-2-oxiranyl-1-benzopyran 2-Chloro-1-(6-fluoro-1-benzopyran-2-yl)-ethanol (2.5 g, 9.20 mmol, 84.9% A) was dissolved in i-PrOH (25 ml) under nitrogen and the reaction mixture cooled to 0° C. A 2M aqueous solution of NaOH (12.5 ml) was added to the solution over 5 min and the reaction was stirred for 1 hour 30 min. The reaction mixture was then diluted with toluene (50 ml) and the pH corrected with acetic acid (0.92 g). Toluene (50 ml) and demineralized water (10 ml) were then added to the mixture and the phases separated after extraction. The collected organic phases were then washed with demineralized water (50 ml). The toluene phase was then anhydrified by azeotropic distillation and concentrated until dryness in a rotary evaporator to give 6-Fluoro-2-oxiranyl-1-benzopyran as a mixture of diastereoisomers 52:48 (2.0 g, 96% yield, 86.1% A). Diast. RR,SS: δH (400 MHz; CDCl3) 6.81-6.72 (3H, m), 3.88-3.82 (1H, m), 3.21-3.17 (1H, m), 2.89-2.76 (4H, m), 2.1-2.00 (1H, m), 1.97-1.87 (1H, m); Diast. SR,SR: δH (400 MHz; CDCl3) 6.84-6.73 (3H, m), 3.87-3.81 (1H, m), 3.15-3.10 (1H, m), 2.91-2.78 (4H, m), 2.18-2.10 (1H, m), 1.96-1.84 (1H, m).
References:
US2011/237808,2011,A1 Location in patent:Page/Page column 5-6
![1-[(2S)-6-fluoro-3,4-dihydro-2H-chromen-2-yl]ethane-1,2-diol](/CAS/20180601/GIF/1322623-11-9.gif)
1322623-11-9
0 suppliers
inquiry

99199-90-3
109 suppliers
inquiry
874649-82-8
92 suppliers
$9.00/250mg

74-97-5
0 suppliers
$15.00/25g

99199-90-3
109 suppliers
inquiry