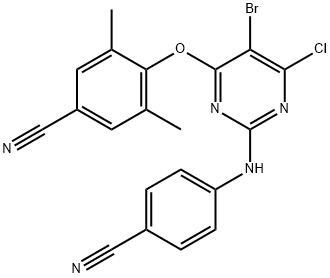
6-Desamino 6-Chloro Etravirine synthesis
- Product Name:6-Desamino 6-Chloro Etravirine
- CAS Number:269055-76-7
- Molecular formula:C20H13BrClN5O
- Molecular Weight:454.71
Yield:269055-76-7 45%
Reaction Conditions:
Stage #1: 3,5-dimethyl-4-hydroxybenzonitrilewith sodium hydride in 1,4-dioxane; for 0.0333333 h;
Stage #2: with 1-methyl-pyrrolidin-2-one in 1,4-dioxane; for 0.166667 h;
Stage #3: 4-[[(5-bromo-4,6-dichloro)-2-pyrimidinyl]amino]benzonitrile in 1,4-dioxane at 155; for 16 h;
Steps:
2
A mixture of 0.47 g of 4-hydroxy-3,5-dimethylbenzonitrile (0.00320 mol) and 1,4-dioxane (3 ml) were added to a pressure tube under argon gas. 0.13 g of NaH 60% (0.00320 mol) was added, and the mixture was stirred for 2 minutes. l-Methyl-2- pyrrolidinone (3 ml) was added and the mixture was stirred for 10 minutes. Intermediate 1 (0.00291 mol) was added and the mixture was heated in a sealed tube at 155°C for 16 hours. The mixture was poured into water (15 ml). The tube was washed with water, 1,4-dioxane (11 ml) and again with water and the washings were combined with the water phase into which the reaction mixture had been poured. The thus obtained solution was stirred for 15 minutes and placed in the refrigerator. The resulting material was filtered to obtain 1.43 g of 4-[[5-bromo-6-chloro-2-[(4-cyano- phenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethylbenzonitrile,yield 45 % (Intermediate 2).
References:
WO2006/94930,2006,A1 Location in patent:Page/Page column 19
![BENZONITRILE, 4-[(4,6-DICHLORO-2-PYRIMIDINYL)AMINO]-](/CAS/GIF/329187-59-9.gif)
329187-59-9
113 suppliers
inquiry

269055-76-7
25 suppliers
inquiry
![4-[(4,6-Dihydroxy-2-pyrimidinyl)amino]benzonitrile](/CAS2/GIF/374067-80-8.gif)
374067-80-8
49 suppliers
inquiry

269055-76-7
25 suppliers
inquiry
![BENZONITRILE, 4-[(5-BROMO-4,6-DICHLORO-2-PYRIMIDINYL)AMINO]-](/CAS/GIF/269055-75-6.gif)