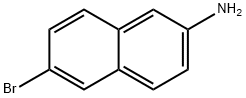
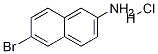
6-Bromonaphthalen-2-amine synthesis
- Product Name:6-Bromonaphthalen-2-amine
- CAS Number:7499-66-3
- Molecular formula:C10H8BrN
- Molecular Weight:222.08
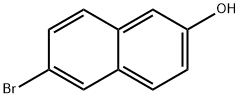
15231-91-1
412 suppliers
$7.00/5g

7499-66-3
223 suppliers
$19.00/1g
Yield:7499-66-3 94%
Reaction Conditions:
with ammonium bisulfite;ammonia;water at 150; for 48 h;
Steps:
6-bromonaphthalen-2-amine (15)6-Bromonaphthalen-2-ol (1.5 g, 6.7 mmol) was heated with ammonium hydroxide (10ml) and ammonium sulfite (3.5 g, 26 mmol) in a seal tube at 150 0C for 48 h. After cooling to room temperature, ethyl acetate was added and organic layer was separated, washed with brine, dried over anhydrous sodium sulfate and evaporated to obtain crude product 15 (1.4 g, Y; 94 %), which was pure enough and can be used directly for next step without further purification. 1H NMR (CDCl3): δ 7.84 (IH, br), 7.56 (IH, d, J= 9.6 Hz), 7.43 (2H, m), 6.95 (2H, m), 3.87 (2H, b). Leigh C. Anderson and Donald G. Thomas: Quinoidation of Triaryl Compounds - Hydroxynaphthyldiphenylcarbinols J. Am. Chem. Soc; 65; 1943; 239, 241.
References:
THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA WO2008/124812, 2008, A1 Location in patent:Page/Page column 53
5773-80-8
310 suppliers
$10.00/1g

7499-66-3
223 suppliers
$19.00/1g
869114-68-1
42 suppliers
$205.00/1g

7499-66-3
223 suppliers
$19.00/1g
71590-31-3
68 suppliers
$9.00/250mg

7499-66-3
223 suppliers
$19.00/1g

13720-04-2
4 suppliers
inquiry

7499-66-3
223 suppliers
$19.00/1g