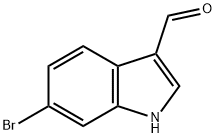
6-Bromoindole-3-carboxaldehyde synthesis
- Product Name:6-Bromoindole-3-carboxaldehyde
- CAS Number:17826-04-9
- Molecular formula:C9H6BrNO
- Molecular Weight:224.05
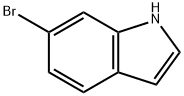
52415-29-9
413 suppliers
$9.00/1g
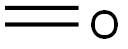
50-00-0
879 suppliers
$10.00/25g

17826-04-9
181 suppliers
$10.00/250mg
Yield:17826-04-9 73%
Reaction Conditions:
with iron(III) chloride;ammonia in water;N,N-dimethyl-formamide at 130; for 5 h;
Steps:
Indolecarbaldehydes 2a-2aa; General Procedure
General procedure: A 50 mL round-bottomed flask equipped with a magnetic stirringbar was charged with the appropriate indole 1 (0.5 mmol,1.0 equiv), 37% aq HCHO (0.5 mmol, 0.0406 g, 1.0 equiv), 25% aqNH3 (1.0 mmol, 0.0681 g, 2.0 equiv), FeCl3 (0.01 mmol, 0.0016 g,2 mol%), and DMF (2 mL). The flask was fitted with a reflux condenser,and the mixture was stirred at 130 °C under open air.When the reaction was complete (TLC), the mixture was cooledto r.t., diluted with sat. aq NaCl (10 mL) and 0.5 M aq HCl (2 mL),and extracted with EtOAc (3 x 7 mL). The organic layers werecombined, washed with sat. aq NaHCO3 (10 mL) and sat. aq NaCl(10 mL), dried (Na2SO4), and concentrated under reduced pressure.The residue was purified by flash column chromatography(silica gel, hexane-EtOAc).
References:
Wang, Qing-Dong;Zhou, Bin;Yang, Jin-Ming;Fang, Dong;Ren, Jiangmeng;Zeng, Bu-Bing [Synlett,2017,vol. 28,# 19,p. 2670 - 2674] Location in patent:supporting information

52415-29-9
413 suppliers
$9.00/1g
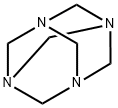
100-97-0
0 suppliers
$14.00/250G

17826-04-9
181 suppliers
$10.00/250mg

52415-29-9
413 suppliers
$9.00/1g
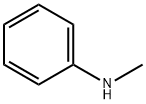
100-61-8
401 suppliers
$8.00/1g

17826-04-9
181 suppliers
$10.00/250mg
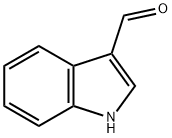
487-89-8
614 suppliers
$6.00/10g
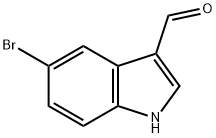
877-03-2
234 suppliers
$6.00/1g

17826-04-9
181 suppliers
$10.00/250mg
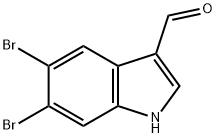
17900-95-7
23 suppliers
inquiry
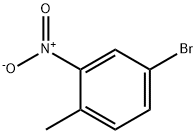
60956-26-5
291 suppliers
$8.00/10g

17826-04-9
181 suppliers
$10.00/250mg