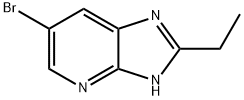
6-Bromo-2-ethyl-3H-imidazo[4,5-b]pyridine synthesis
- Product Name:6-Bromo-2-ethyl-3H-imidazo[4,5-b]pyridine
- CAS Number:68175-12-2
- Molecular formula:C8H8BrN3
- Molecular Weight:226.07
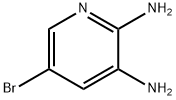
38875-53-5
359 suppliers
$6.00/1g
802294-64-0
1 suppliers
inquiry
![6-Bromo-2-ethyl-3H-imidazo[4,5-b]pyridine](/CAS/GIF/68175-12-2.gif)
68175-12-2
12 suppliers
$44.00/250mg
Yield:68175-12-2 70%
Reaction Conditions:
with ammonium chloride;trichlorophosphate at 100; for 24 h;
Steps:
3 4.1.2.1 General procedures A and B for the synthesis of 3H-imidazo[4,5-b]pyridines from 2,3-diaminopyridines
General procedure: A: A mixture of 2,3-diaminopyridine (1 equiv.), NH4Cl (6 equiv.) and the corresponding carboxylic acid (1.5 equiv.) in POCl3 (0.6M) was heated at 100°C for 24h. The mixture was subsequently poured on ice and neutralized with 3M NaOH aqueous solution. The aqueous layer was extracted with EtOAc and the combined organic layers were dried on MgSO4 and concentrated under reduced pressure. The residue was purified by chromatography eluting with 0 to 10 % EtOH in DCM gradient to give the corresponding product.
References:
Baladi, Tom;Aziz, Jessy;Dufour, Florent;Abet, Valentina;Stoven, Véronique;Radvanyi, Fran?ois;Poyer, Florent;Wu, Ting-Di;Guerquin-Kern, Jean-Luc;Bernard-Pierrot, Isabelle;Garrido, Sergio Marco;Piguel, Sandrine [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 20,p. 5510 - 5530]