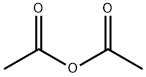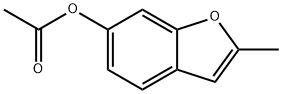
6-acetoxy-2-methylbenzofuran synthesis
- Product Name:6-acetoxy-2-methylbenzofuran
- CAS Number:92810-82-7
- Molecular formula:C11H10O3
- Molecular Weight:190.2
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 16 h;
Steps:
33.2
Step 2 Acetic acid 2-methyl-beriznf]τfiτi-6-yl esterA solution of 6-methoxy-2-methyl-benzofuran (17.4 g, 107 mmol) in dichloromethane (200 mL) at 0 0C is treated with boron tribromide (1.0 M, 107 mL). The mixture is stirred at 0 0C for 60 minutes and quenched with water (50 mL). The organic layer is dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product is purified by flash chromatography eluting with 25% EtOAc/Hexanes. The appropriate fractions are concentrated under reduced pressure. The resulting material is dissolved in dichloromethane (150 mL) and triethylamine (17.0 mL, 122 mmol) at 0 0C and treated with acetic acid anhydride (7.22 mL, 76.35 mmol). The reaction is stirred for 16 hours and allowed to warm to room temperature. The reaction is quenched with MeOH (10 mL) and concentrated under reduced pressure. The residue is purified by silica gel chromatography eluting with 25% EtOAc/Hexanes to provide the title compound (9.50 g, 82%). 1HNMR (400 MHz, CDCl3): δ 7.40-7.38 (d, IH), 7.15 (s, IH), 6.91-6.88 (d, IH), 6.32 (s, IH), 2.41 (s, 3H), 2.29 (s, 3H).
References:
WO2007/92751,2007,A2 Location in patent:Page/Page column 27
54584-24-6
45 suppliers
inquiry
![Benzo[b]thiophene-6-ol, 2-methyl-, 6-acetate](/CAS/20200515/GIF/638216-96-3.gif)
638216-96-3
0 suppliers
inquiry
75-36-5
579 suppliers
$17.92/100G

92810-82-7
2 suppliers
inquiry