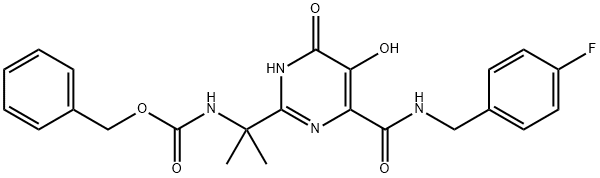
Benzyl (2-(4-((4-fluorobenzyl)carbamoyl)-5-hydroxy-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)carbamate synthesis
- Product Name:Benzyl (2-(4-((4-fluorobenzyl)carbamoyl)-5-hydroxy-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)carbamate
- CAS Number:519028-33-2
- Molecular formula:C23H23FN4O5
- Molecular Weight:454.45
![4-PYRIMIDINECARBOXYLIC ACID, 1,6-DIHYDRO-5-HYDROXY-2-[1-METHYL-1-[[(PHENYLMETHOXY)CARBONYL]AMINO]ETHYL]-6-OXO-, METHYL ESTER](/CAS/GIF/519032-08-7.gif)
519032-08-7
164 suppliers
$10.00/1g
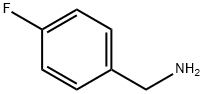
140-75-0
472 suppliers
$10.00/10g

519028-33-2
23 suppliers
inquiry
Yield:519028-33-2 98%
Reaction Conditions:
with triethylamine in methanol at 65 - 68; for 7 h;
Steps:
1.1
A round bottom flask was charged with methyl 2-(l-{[(benzyloxy)carbonyl]amino}-l-methylethyl)-5,6-dihydroxypyrimidine-4-carboxylate (Compound I; 1 eq.) and methanol (-1.6 mL per gram of Compound 1). The slurry was warmed to 55°C, after which triethylamine (1.2 eqs.) was added in one portion. The solution was then warmed to 65°C and 4-fluorobenzylamine (1.2 eqs) was added at 65-68°C. The mixture was then aged at reflux for 7 hours. The solution was cooled to 550C5 and acetic acid (2 eqs.) was added in one portion. Water was added followed by seed crystals of 2. (Note: Crystallization would occur without seed, but seeding provides a more reliable method of crystal growth.) The resultant slurry was aged at 6O0C with the addition of more water during the ageing. The slurry was then cooled to 2O0C5 filtered, washed with 1 : 1 methanol:water, and dried in a nitrogen stream to give compound 2 (96 wt.% purity by HPLC assay, 1.75 wt.% water by Karl Fisher titration), 98% overall yield (corrected for purity). Compound 2: lH NMR (600.13 MHz, CDCI3) δ 12.37 (br s, 2H), 7.95 (br t, J~6 Hz, IH), 735-7.31 (m, 2H)57.25-7.19 ( br m, 5H), 7.08-7.04 (m, 2H), 6.49 (s, IH)5 4.96 (s, 2H)5 4.58 (d, 3=6.0 Hz, 2H)5 1.64 (s, 6H).13C NMR (150.92 MHz, CDCI3) δ 168.5, 162.6 (d, JcF=246.6 Hz), 160.O5 155.35 154.1, 147.9, 136.5, 133.1 (d, JCF=3-1 Hz), 129.7 (d, JCF^7.9 Hz), 128.6, 128.1, 127.9, 127.1, 116.0 (d5 JCF=22.0 Hz), 66.8, 55.5, 42.7, 26.7.
References:
WO2009/88729,2009,A1 Location in patent:Page/Page column 44
![BENZYL [1-CYANO-1-METHYLETHYL]CARBAMATE](/CAS/GIF/100134-82-5.gif)
100134-82-5
80 suppliers
inquiry

519028-33-2
23 suppliers
inquiry
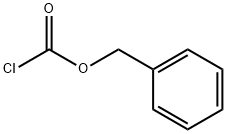
501-53-1
398 suppliers
$10.00/1g

519028-33-2
23 suppliers
inquiry
![BENZYL [2-AMINO-2-(HYDROXYIMINO)-1,1-DIMETHYLETHYL]CARBAMATE](/CAS/GIF/518047-98-8.gif)
518047-98-8
58 suppliers
$120.00/1g

519028-33-2
23 suppliers
inquiry
![2-Butenedioic acid, 2-[[[1-imino-2-methyl-2-[[(phenylmethoxy)carbonyl]amino]propyl]amino]oxy]-, 1,4-dimethyl ester](/CAS/20210305/GIF/1064707-10-3.gif)
1064707-10-3
0 suppliers
inquiry

519028-33-2
23 suppliers
inquiry