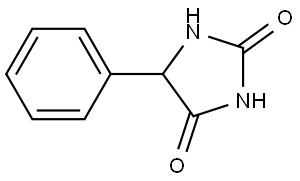
5-Phenylhydantoin synthesis
- Product Name:5-Phenylhydantoin
- CAS Number:89-24-7
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
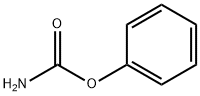
622-46-8
98 suppliers
$33.20/5g
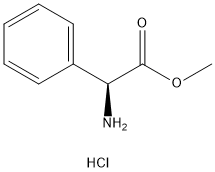
15028-39-4
215 suppliers
$5.00/1g

89-24-7
121 suppliers
$14.14/250mgs:
Yield: 73%
Reaction Conditions:
Stage #1:carbamic acid phenyl ester;L-2-phenylglycine methyl ester hydrochloride with triethylamine in acetonitrile for 10 h;Reflux;
Stage #2: with sodium hydroxide in acetonitrile for 8 h;Reflux;
Steps:
General procedure for synthesis of substituted hydantoins (3a-w)
General procedure: α-amino methyl ester hydrochloride (2 mmol) and carbamate (2.2 mmol) were dissolved in amixture of acetonitrile (12 ml) and triethylamine (6 ml) and reaction mixture was refluxed for 10h. Then, NaOH (5 mmol) was added and reaction was continued for another 8 hours. After thecompletion of the reaction, solvents were distilled off, and the residue was partitioned betweenethyl acetate and 0.1N aqueous HCl and extracted. The organic layer was washed with brine followed by drying over anhydrous Na2SO4. The concentration of the organic layer gave thecrude product which was purified either by recrystallization (hexane-ethyl acetate mixture) or bycolumn chromatography (hexane:ethylacetate) to afford the desired product.
References:
Tanwar, Dinesh Kumar;Ratan, Anjali;Gill, Manjinder Singh [Synlett,2017,vol. 28,# 17,art. no. ST-2017-D0256-L,p. 2285 - 2290] Location in patent:supporting information
201230-82-2
1 suppliers
inquiry
100-52-7
971 suppliers
$17.30/2g
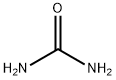
57-13-6
748 suppliers
$5.00/5g

89-24-7
121 suppliers
$14.14/250mgs:
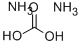
506-87-6
387 suppliers
$9.00/25g

100-52-7
971 suppliers
$17.30/2g

89-24-7
121 suppliers
$14.14/250mgs:
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

89-24-7
121 suppliers
$14.14/250mgs:
201230-82-2
1 suppliers
inquiry

100-52-7
971 suppliers
$17.30/2g

57-13-6
748 suppliers
$5.00/5g

89-24-7
121 suppliers
$14.14/250mgs:
ACETIC ACID](/StructureFile/ChemBookStructure21/GIF/CB2839563.gif)
82264-50-4
8 suppliers
inquiry